Quantum chemistry (QC) is crucial for understanding protein-ligand interactions, but its computations are often too costly. Machine-learned potentials offer a solution, but struggle to capture long-range, non-local interactions. To address this, researchers have developed an atomic-pairwise neural network (APNet) for modeling intermolecular interactions. The APNet model, which predicts interaction energies via an intermediate prediction of monomer electron densities, benefits from physical constraints and a comprehensive training dataset. It accurately predicts QC-quality interaction energies of protein-ligand systems at a significantly reduced computational cost. This development could have profound implications for drug design and other chemical sciences.
What is the Role of Quantum Chemistry in Understanding Protein-Ligand Interactions?
Quantum chemistry (QC) plays a significant role in understanding protein-ligand interactions, which are crucial in many chemical problems. However, QC computations on protein-ligand systems are often too computationally expensive for most use cases. The field of machine-learned (ML) potentials offers a promising solution, but it is limited by an inability to easily capture long-range, non-local interactions.
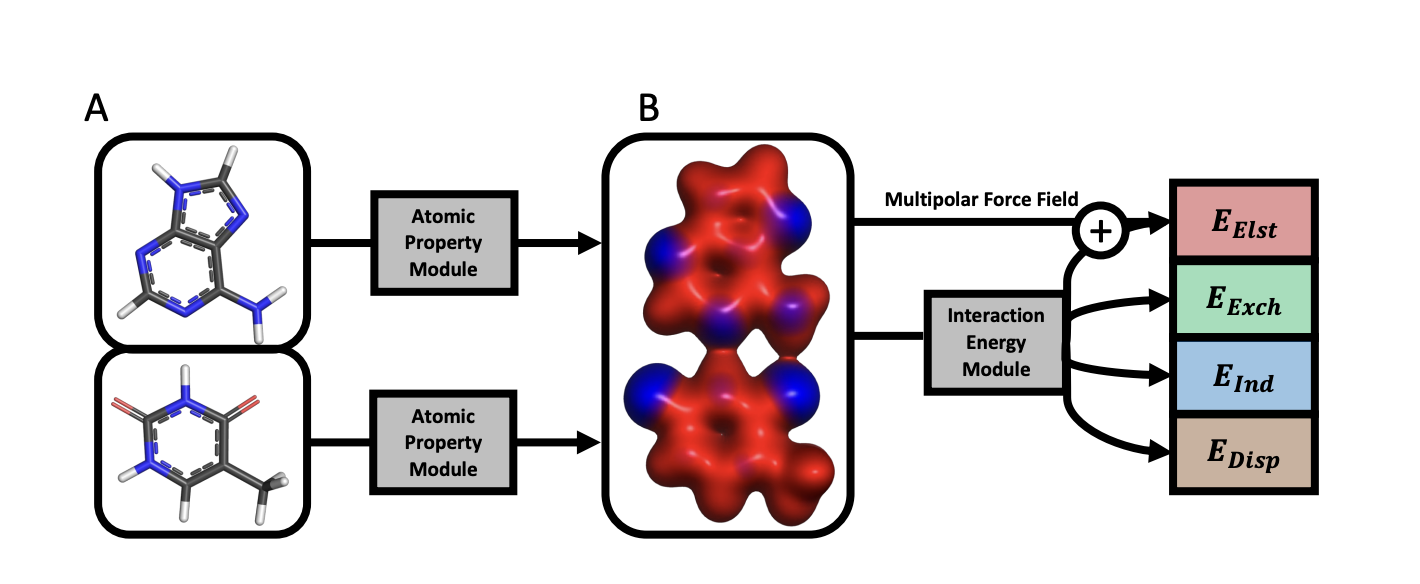
In this context, a team of researchers has developed an atomic-pairwise neural network (APNet) specialized for modeling intermolecular interactions. This model benefits from a number of physical constraints, including a two-component equivariant message passing neural network architecture that predicts interaction energies via an intermediate prediction of monomer electron densities. The APNet model also benefits from a comprehensive training dataset composed of paired ligand and protein fragments. This model accurately predicts QC-quality interaction energies of protein-ligand systems at a computational cost reduced by orders of magnitude.
How Do Noncovalent Interactions Influence Chemical and Biomolecular Systems?
Noncovalent interactions (NCIs) play a key role in the chemical sciences. Although they are weaker than covalent bonds, the presence or absence of NCIs can have profound effects on chemical and biomolecular systems. For example, NCIs drive DNA intercalation—the insertion of a molecule between consecutive DNA base pairs—which is the mechanism of action in anticancer drugs such as doxorubicin.
NCIs also play a particularly important role in small molecule drug design. The efficacy of a drug depends in part on the presence of strong interactions with the target protein. Often, preliminary drug design efforts produce a promising but suboptimal lead compound. This lead compound is then iteratively revised in an attempt to enhance desired properties. Maximizing favorable intermolecular contacts is a critical aspect of the lead optimization process and can be aided by in silico models of NCIs.
How Does Quantum Chemistry Quantify Protein-Ligand Interactions?
The strength of a protein-ligand interaction can be rigorously quantified by using the tools of quantum chemistry to compute an interaction energy. Quantum chemistry methods, which seek to solve the many-body Schrödinger equation, are subject to a well-established trade-off between computational cost and accuracy.
Highly accurate interaction energies can be obtained from wavefunction-based methods such as coupled cluster (CC) theory, but such calculations are often too expensive for all but the smallest systems. Less expensive methods like density functional theory (DFT) yield slightly less robust interaction energies. Simple transferable force fields like GAFF are orders of magnitude faster than any quantum chemistry method, but force field interaction energies are only semi-quantitatively accurate.
What is the Role of Machine Learning in Quantum Chemistry?
Recently, advances in machine learning (ML) have led to the reevaluation of this classic cost-accuracy tradeoff. Models like neural networks (NNs) are capable of expressing arbitrarily complicated nonlinear functions such as molecular potential energy surfaces. Large datasets of quantum chemical computations make it possible to parameterize general atomistic NN potentials to quantum chemical accuracy, and these potentials can be evaluated at near force field computational cost.
The emergence of NN potentials has also benefited from a number of architectural developments. The message passing NN (MPNN) framework is tailored to the graph-like structure of molecular geometries. Directional MPNNs additionally account for the relative orientation between neighboring atoms in a molecule. The most data-efficient architectures achieve this by employing locally equivariant representations of atoms in molecules.
How Does the Atomic-Pairwise Neural Network Overcome Limitations?
The locality of atomic environments used in NN potentials allows these models to easily describe local, mostly covalent interactions like distortions of bonds, angles, and dihedrals. Unfortunately, a consequence of this locality is that NN potentials are either indirectly or explicitly unable to model the long-range, non-local interactions that are crucial in protein-ligand systems.
To overcome this limitation, the researchers developed the atomic-pairwise neural network (APNet), which is specialized for modeling intermolecular interactions. The APNet model benefits from a number of physical constraints, including a two-component equivariant message passing neural network architecture that predicts interaction energies via an intermediate prediction of monomer electron densities. The APNet model also benefits from a comprehensive training dataset composed of paired ligand and protein fragments. This model accurately predicts QC-quality interaction energies of protein-ligand systems at a computational cost reduced by orders of magnitude.
Publication details: “A Physics-Aware Neural Network for Protein-Ligand Interactions with Quantum Chemical Accuracy”
Publication Date: 2024-02-16
Authors: Zachary Glick, Derek Metcalf, Caroline Sargent, Steven A. Spronk et al.
Source:
DOI: https://doi.org/10.26434/chemrxiv-2024-5v6gh