Electrochemical electron transfer underpins many crucial technologies, from batteries to fuel cells, yet accurately modelling this process at a fundamental level remains a significant challenge. Mengke Zhang, Yanxia Chen, Marko M Melander, and Jun Huang present a comprehensive review of the key concepts and theories governing electron transfer kinetics at electrochemical interfaces. Their work elucidates the interplay between classical solvent behaviour and electronic states, offering a detailed exploration of weak, strong, and intermediate electronic coupling regimes. By highlighting the power of atomistic simulations, particularly density functional theory and molecular dynamics, the researchers demonstrate how these methods can determine critical parameters such as solvent reorganization energy and electronic coupling strengths, ultimately advancing our understanding and predictive capabilities in this vital field.
Electrocatalysis And The Electric Double Layer
Research focuses on understanding the fundamental processes governing electrochemical reactions, such as carbon dioxide reduction and hydrogen evolution, with a central emphasis on the electric double layer, the interface between an electrode and an electrolyte. This region is critical, as it dictates the conditions for electrochemical activity. Scientists are investigating how water’s dielectric properties change within this confined space, leading to non-local effects that influence reaction rates. The arrangement and interactions of ions and solvent molecules within the electric double layer are also under scrutiny, with researchers exploring short-range correlations and specific ion effects.
Understanding the nature of surface charge and capacitance, complex and influenced by the electric double layer’s structure, is also a key area of investigation. Researchers are exploring different models to accurately describe how charge is distributed at the interface. Furthermore, scientists are delving into the details of how electrons move during electrochemical reactions, examining whether electron transfer occurs smoothly or abruptly, and how energy is transferred between molecules during the process. They are considering both quantum and classical descriptions of electron transfer to gain a complete understanding.
A significant portion of this research relies on computational methods, including density functional theory, which calculates electronic structure, and molecular dynamics, which simulates the movement of atoms and molecules. Researchers are employing both implicit and explicit solvent models to represent the solvent environment in their simulations, and combining density functional theory with other methods to improve accuracy. This work aims to develop a detailed, molecular-level understanding of electrochemical reactions, with the ultimate goal of designing more efficient and selective catalysts and energy storage devices.
Semiclassical Kinetics of Electrochemical Interface Electron Transfer
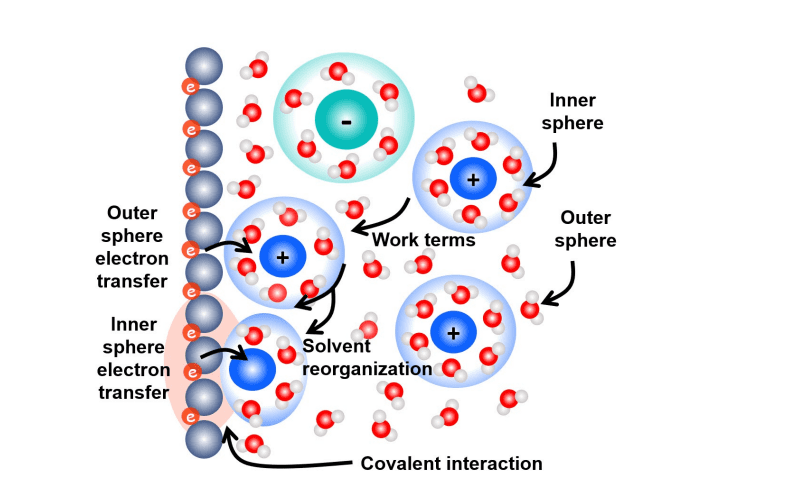
Scientists are investigating electron transfer at electrochemical interfaces, a process central to energy conversion and storage, by employing a combination of theoretical and computational techniques. This work pioneers a semiclassical framework that integrates classical solvent dynamics with quantum electronic states to understand the kinetics of electron transfer reactions. Researchers establish the fundamental rate constant for electron transfer, dependent on solvent fluctuations, free energy barriers, and the electronic transmission coefficient, and meticulously derive the key equations governing this process. To accurately model the complex interfacial region, known as the electrical double layer, the team utilizes density functional theory and molecular dynamics simulations.
These computational methods enable precise determination of the solvent reorganization energy, a crucial parameter influencing the rate of electron transfer, and allow for mapping both diabatic and adiabatic free energy surfaces. Density functional theory calculations characterize the electronic structure of the electrode-solution interface, while molecular dynamics simulations capture the dynamic behavior of solvent molecules surrounding redox species. Scientists are refining their approach by examining the limitations of linear response approximations, commonly used to simplify complex calculations, particularly in scenarios involving strong solvation changes or inner-sphere electron transfer. To overcome these limitations, the team develops advanced methodologies, including mapping Hamiltonian-based empirical valence bond molecular dynamics, constrained density functional theory, and non-Gaussian free energy formulations. These techniques allow for rigorous testing of linear response and provide access to detailed information about the electronic coupling strength between the metal surface and redox species, as well as the frequency of solvent nuclear fluctuations. The study demonstrates how these combined computational and theoretical methods provide a comprehensive understanding of electron transfer kinetics, essential for optimizing the performance of energy conversion and storage devices.
Solvent Dynamics Control Electron Transfer Kinetics
Scientists have achieved a detailed understanding of electron transfer, a fundamental process in energy conversion and storage, by meticulously examining the interplay between solvent dynamics and electronic states at electrochemical interfaces. This work establishes a framework for modeling electron transfer kinetics, focusing on how solvent fluctuations and electronic interactions govern the rate of these reactions. The research demonstrates that the efficiency of electron transfer is critically dependent on the strength of electronic coupling between reacting species, revealing two distinct regimes: non-adiabatic and adiabatic electron transfer. Experiments reveal that in the non-adiabatic regime, characterized by extremely weak electronic coupling, electron transfer is slow and occurs at the intersection of diabatic free energy surfaces, with a high probability of excitation to an excited state and a very small electron transfer coefficient.
Conversely, the team discovered that sufficiently strong electronic coupling leads to adiabatic electron transfer, where the electron follows the solvent nuclei on the ground state, resulting in an electron transfer coefficient close to unity. This strong coupling, observed in electrocatalytic reactions, facilitates the formation of a covalent bond between the metal surface and reacting species, significantly reducing activation energy. Measurements confirm that the timescale for electron transfer is determined by comparing the rates of electronic and nuclear motion in the transition region. The team established that stronger electronic coupling leads to a smaller timescale for electronic motion, allowing the electron to adjust more quickly to follow nuclear motion and increasing the probability of transitioning along the ground state free energy surface.
Furthermore, the research highlights the importance of inner-sphere electron transfer, where significant distortion occurs in the inner solvation shell of the reacting species, as opposed to outer-sphere transfer, which involves minimal changes to the inner solvation shell. Building on earlier theoretical work, the team revisited foundational concepts in electron transfer theory, including the application of the Franck-Condon principle and the development of non-equilibrium polarization theory. This work provides a comprehensive framework for understanding and modeling electron transfer processes, paving the way for advancements in energy storage technologies and electrocatalysis.
Atomistic Simulations Illuminate Electron Transfer Kinetics
This review presents a comprehensive overview of electron transfer kinetics at electrochemical interfaces, integrating theoretical derivations with insights from atomistic simulations. Researchers systematically explored the fundamental principles governing electron transfer, beginning with continuum electrostatic descriptions of solvent fluctuations and extending to quantum mechanical treatments of transitions between electronic states. The work establishes a conceptual framework linking solvent reorganization, electronic coupling strength, and solvent dynamics to electrochemical electron transfer phenomena. A key achievement is demonstrating how parameters essential to electron transfer theory, reorganization energy, electronic coupling, and solvent nuclear frequency, can be determined using atomistic simulations.
The review also highlights the crucial role of the electrical double layer in influencing electron transfer rates through changes in work terms, reorganization energy, and concentrations. Throughout this investigation, the central assumption of linear response between electron transfer and solvent response was emphasized, enabling simplified equations and facilitating simulations. However, the authors acknowledge that the validity of this linear response approximation is not always guaranteed, particularly in electrocatalytic or inner-sphere electron transfer reactions where substantial structural changes occur within the solvent. Future work, they suggest, requires moving beyond this linear response approximation to accurately model reactions involving significant changes in solvation.
👉 More information
🗞 Electrochemical Electron Transfer: Key Concepts, Theories, and Parameterization via Atomistic Simulations
🧠 ArXiv: https://arxiv.org/abs/2510.24635