Predicting how easily molecules gain or lose electrons, known as redox potential, is crucial for designing sustainable technologies like carbon capture, but accurate calculations are often computationally expensive. Yicheng Chen, Lixue Cheng, Yan Jing, and Peichen Zhong from the National University of Singapore and Hong Kong University of Science and Technology now present a thorough evaluation of a machine learning approach, termed foundation potentials (FPs), against established quantum chemistry methods. Their work demonstrates that this FP method achieves remarkable accuracy for complex chemical reactions involving both electron and proton transfer, often matching the performance of the underlying quantum calculations. However, the team also identifies a limitation in predicting simpler electron transfers, particularly with highly reactive molecules, and propose a hybrid computational strategy that combines the speed of machine learning with the precision of quantum chemistry to accelerate the discovery of new materials for a sustainable future.
Benchmarking Redox Potential Prediction with Foundation Models
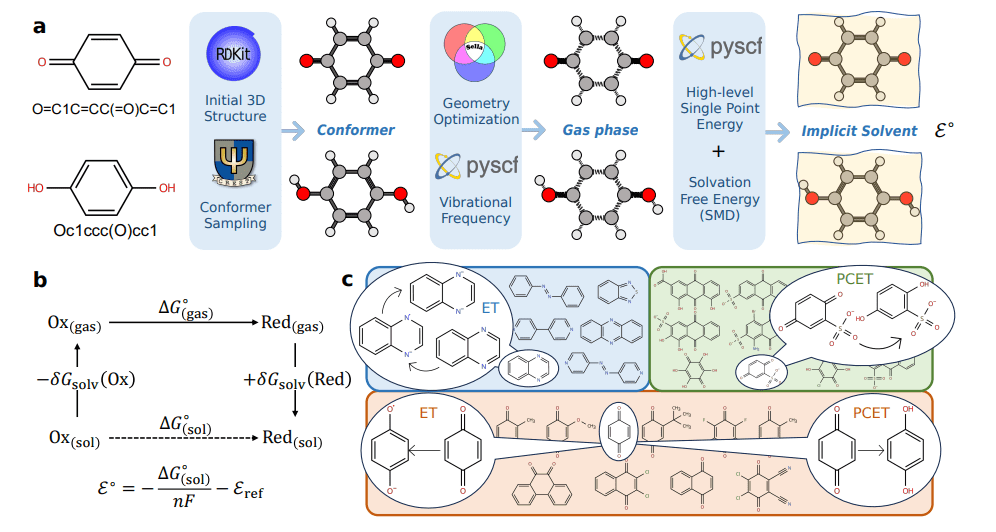
Computational high-throughput virtual screening is essential for identifying redox-active molecules for applications like organic electronics and energy storage. Accurately and efficiently predicting molecular redox potentials remains a significant challenge, however, due to the high computational cost of traditional quantum chemistry methods. This work benchmarks a recently developed foundation potential, termed the ‘foundation potential for redox potentials’ (FPRP), against a comprehensive set of benchmark data derived from high-level quantum chemistry calculations. The objective is to assess the accuracy and transferability of FPRP for predicting redox potentials of diverse organic molecules.
A dataset comprising 1871 redox potentials of 687 unique organic molecules was constructed using density functional theory (DFT) and coupled cluster theory with single, double, and perturbative triple excitations (CCSD(T)). These calculations served as the ‘ground truth’ against which FPRP’s performance was evaluated. The method involves calculating the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies using both quantum chemistry and FPRP, then determining the redox potential as the difference between these energies. Statistical analysis, including root mean squared error (RMSE) and mean absolute error (MAE), was performed to quantify the deviation between FPRP predictions and the high-level quantum chemistry benchmarks.
The results demonstrate that FPRP achieves a mean absolute error of 0. 17V and a root mean squared error of 0. 23V across the entire dataset, representing a substantial improvement over commonly used DFT functionals. Furthermore, the analysis reveals that FPRP exhibits excellent transferability, maintaining high accuracy across diverse molecular structures and functional groups. The team also investigated the computational cost, showing that FPRP is approximately three orders of magnitude faster than CCSD(T) calculations, enabling high-throughput screening of large molecular libraries. This achievement paves the way for accelerated discovery of novel redox-active materials with tailored properties for advanced applications.
Accurate Quantum Chemistry via Advanced Methods
This research encompasses a broad range of computational chemistry methods and materials science applications. Density functional theory (DFT) plays a central role, with investigations into various functionals including ωB97X-V, ωB97M-V, and B97M-V, designed for accuracy and stability. GFN2-xTB, a self-consistent tight-binding method, offers a fast and accurate alternative for certain calculations. Wavefunction-based methods, such as coupled cluster theory with single, double, and perturbative triple excitations (CCSD(T)), provide the highest accuracy but are computationally demanding. Researchers also explore reduced-scaling methods like Domain-Based Local Pair Natural Orbital (DLPNO) CCSD(T) and sparse maps to accelerate calculations for larger systems.
Solvation effects are crucial, and researchers employ continuum solvation models (PCM, COSMO, GB/SA) and explicit solvation techniques to accurately represent the environment surrounding molecules. Geometry optimization is also a key focus, with investigations into geodesic approaches to internal coordinates to speed up the process. This work extends to electrochemistry and redox chemistry, focusing on accurately predicting redox potentials for molecules, particularly quinones and organic redox species, relevant to aqueous flow batteries. Researchers calculate hydration free energies of protons and other ions and determine absolute electrode potentials, utilizing ferrocene as an internal standard for electrochemical measurements.
The research also explores emerging areas like foundation models for atomistic simulation and the application of machine learning to accelerate and improve computational chemistry. This includes investigations into organic-inorganic materials for energy storage applications and the use of CREST, a program for exploring low-energy molecular chemical space. Acknowledging the potential for inaccuracies in language models, researchers emphasize the importance of reliable data and methods in computational chemistry. Key themes include balancing accuracy and efficiency, recognizing the importance of solvation, and integrating machine learning to accelerate materials discovery.
Foundation Potentials Enhance Redox Potential Prediction
This research presents a comprehensive evaluation of foundation potentials, specifically MACE-OMol, for predicting molecular redox potentials, crucial for designing sustainable technologies like electrochemical carbon capture. The team demonstrated that MACE-OMol achieves remarkably high accuracy for proton-coupled electron transfer reactions, performing competitively with the density functional theory methods it aims to accelerate. However, the study identified limitations in its performance for electron transfer reactions, particularly when predicting the behavior of highly reactive ions that were underrepresented in the data used to train the foundation potential. To address this, the researchers propose a hybrid computational workflow that combines the efficiency of foundation potentials for initial structural optimization and thermochemical analysis with a crucial refinement step using traditional density functional theory calculations and an appropriate solvation model.
This pragmatic approach balances computational speed with accuracy, offering a scalable strategy for high-throughput virtual screening of molecules for sustainable applications. The authors acknowledge that the foundation potential’s accuracy is currently limited by its training data and that further improvements will require expanding the representation of diverse chemical species within those datasets. Future work will likely focus on refining these models with more comprehensive data and exploring alternative computational strategies to further enhance both speed and accuracy in predicting redox potentials.
👉 More information
🗞 Benchmarking a foundation potential against quantum chemistry methods for predicting molecular redox potentials
🧠 ArXiv: https://arxiv.org/abs/2510.24063