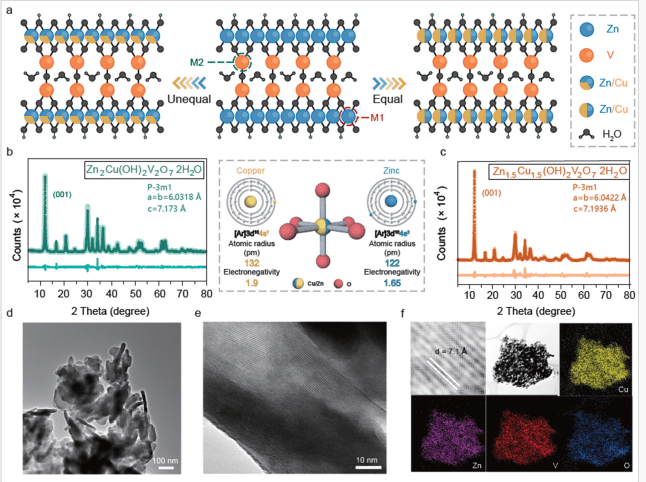
In advancing beyond lithium-ion batteries, researchers have developed a novel approach to enhance calcium-ion storage performance using zinc-centered materials as hosts. By introducing copper into these materials through a scalable co-precipitation method, they created Cu/Zn solid-solution phases with varying ratios, leveraging their similar atomic properties to maintain structural integrity.
The resulting vanadate host’s layered crystal structure provides favourable diffusion pathways for charge carriers, while interlayer confined species such as water and hydroxyl groups further facilitate ion movement. The presence of copper enhances redox activity, improving electrochemical performance in an organic electrolyte system. This study combines theoretical simulations analyzing lattice water’s role in diffusion kinetics with experimental validation, demonstrating the effectiveness of this solid-solution design strategy for multivalent charge carrier hosts.
Next-generation multivalent metal ion batteries
Next-generation multivalent metal ion batteries represent a promising sustainable energy storage technology advancement. These systems utilize ions such as calcium, which can transfer multiple electrons during electrochemical reactions, offering potential advantages over traditional lithium-ion batteries.
However, using multivalent ions presents challenges due to their stronger electrostatic interactions with host materials. This leads to slower diffusion kinetics and limitations in electrochemical performance. Researchers have developed Cu/Zn solid-solution phase hosts to address these issues using hydrated vanadate as a base material. This approach leverages the host material’s layered crystal structure, which provides favorable pathways for charge carrier diffusion.
Introducing active copper into the zinc lattice enhances redox reaction activity, significantly improving calcium-ion storage capacity in organic electrolyte systems. The design strategy employs a scalable co-precipitation method to create solid-solution structures with varying ratios of copper and zinc. This method ensures minimal lattice distortion, maintaining structural integrity and robustness.
Theoretical simulations using first-principles calculations have provided insights into the role of lattice water in charge carrier diffusion. These studies reveal that lattice water facilitates diffusion kinetics and stabilises interlayer structures. Additionally, the solid-solution substitution enhances the electronic conductivity of pyrovanadate, further improving battery performance.
Experimental validation has demonstrated the effectiveness of this approach. The Cu/Zn solid-solution phase materials successfully activate redox reaction plateaus, enabling reversible calcium-ion storage behavior. These results confirm the viability of the proposed design strategy for multivalent metal ion batteries, offering a promising path forward in developing next-generation energy storage solutions.
Design of Cu/Zn solid-solution phase hosts
The design of Cu/Zn solid-solution phase hosts represents a strategic approach to overcoming challenges in multivalent metal ion batteries. Researchers have created a material framework that supports efficient charge carrier diffusion by leveraging the layered crystal structure of hydrated vanadate. Introducing copper into the zinc lattice introduces active sites that enhance redox reaction activity, thereby improving calcium-ion storage capacity.
The scalable co-precipitation method used to synthesize these materials ensures minimal lattice distortion, maintaining structural integrity while allowing for varying ratios of copper and zinc. This approach is underpinned by the similarity in properties between copper and zinc, as both are transition metals in the ds block with comparable atomic radii and electronegativities.
Theoretical simulations have revealed that lattice water plays a critical role in facilitating charge carrier diffusion, while solid-solution substitution stabilizes interlayer structures. These findings underscore the importance of the “water-locking” mechanism in maintaining structural stability and enhancing electronic conductivity.
Experimental validation of calcium-ion storage capability
The experimental validation of calcium-ion storage capability demonstrates the effectiveness of Cu/Zn solid-solution phase materials in enabling reversible storage behavior. Electrochemical testing revealed that these materials successfully activate redox reaction plateaus, confirming their potential for advancing multivalent metal ion batteries.
These results highlight the practical application of the design strategy, offering a solution to challenges associated with slower diffusion kinetics and structural instability. The findings confirm the viability of Cu/Zn solid-solution phase materials in next-generation energy storage systems.
More information
External Link: Click Here For More