The seemingly simple act of mixing gases presents a complex challenge when considered outside the established framework of thermal equilibrium, particularly in isolated systems where energy exchange with an external heat source is absent. Understanding the thermodynamic limits of such processes has implications for various fields, including carbon capture technologies and advanced water purification methods. Now, Budhaditya Bhattacharjee, Rohit Kishan Ray, and Dominik Safranek, alongside their colleagues, investigate this phenomenon in a new study titled ‘Work and entropy of mixing in isolated quantum systems’. Their research identifies the entropy of mixing as a specific instance of ‘observational entropy’, a concept that depends on the precision of measurement. It establishes a link between accessible information and the potential for energy extraction, offering a rigorous framework for quantifying the energy needed to reverse the mixing process in non-equilibrium scenarios.
Efficient mixing and separation underpin a wide range of technologies and environmental solutions. The fundamental process of mixing is ubiquitous and critical to numerous technological applications, ranging from carbon capture to water purification, demanding increasingly efficient solutions as environmental challenges intensify. Understanding the energetic costs associated with both mixing and subsequent separation of substances is therefore paramount, particularly as traditional thermodynamic analyses of mixing rely on the assumption of local thermal equilibrium, where systems readily exchange energy with their surroundings. Contemporary systems frequently operate far from equilibrium, necessitating a re-evaluation of established principles when considering isolated systems or those lacking a connection to a thermal reservoir.
Recent research investigates these phenomena within the framework of isolated systems, where traditional equilibrium assumptions no longer hold, aiming to establish a rigorous framework for determining the energy required to separate mixtures in non-equilibrium settings. By focusing on macroscopic observables, scientists seek to resolve the Gibbs paradox by explicitly incorporating the observer’s resolution of measurement and the resulting impact on both entropy and extractable work, introducing the concept of an “observational temperature”. This novel parameter emerges from the accessible information. It provides a bound on the difference in energy extracted by different observers, extending beyond classical gases and potentially offering insights into more complex systems.
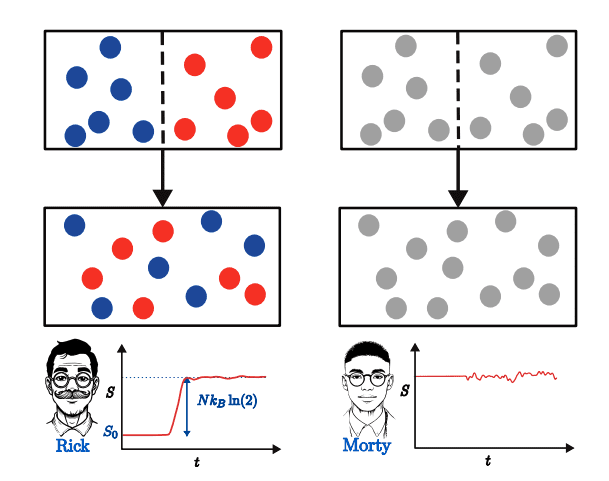
The seemingly simple act of gas mixing receives renewed scrutiny through a thermodynamic framework that prioritises the role of the observer and the limits of measurement. Researchers are moving beyond traditional analyses, which assume systems readily exchange energy with a surrounding heat bath, instead focusing on isolated systems that are potentially far from equilibrium, and fundamentally redefining entropy. This work frames entropy not as an inherent property of the system, but as ‘observational entropy’, dependent on what an observer can measure, recognising that entropy increases not simply because two gases combine, but because an observer learns of their distinct identities.
This observer-dependent perspective elegantly addresses the long-standing Gibbs mixing paradox, clarifying that while an observer registering the presence of two particle types experiences this entropy increase, this knowledge is irrelevant for work extraction if the particles remain operationally indistinguishable through measurement. In essence, knowing the gases are different doesn’t provide a means to harness energy from them unless that difference can be detected and exploited, shifting the focus from the gases themselves to the limitations of the observational apparatus. The methodology employed centres on defining macroscopic observables, effectively sidestepping the need to track individual particle behaviour, allowing researchers to derive a relationship between entropy and the potential for extracting work.
Central to the findings is the derivation of a Landauer-like bound – a principle borrowed from information theory relating energy dissipation to information erasure – applied to the context of gas mixing, quantifying the difference in energy extractable by two observers. The research introduces the concept of an ‘observational temperature’, determined by the amount of accessible information, governing the energy exchange between observers and the system.
The implications of this work extend beyond the confines of simple gas mixtures, as the researchers provide a rigorous method for determining the energy required to unmix gases in non-equilibrium settings, offering insights into the thermodynamics of isolated systems more broadly. By framing thermodynamics within an observer-dependent context, the principles developed are not limited to classical gases, potentially informing our understanding of complex systems where equilibrium is rarely achieved. The emphasis on observational limits and accessible information represents a significant methodological shift, moving beyond purely system-centric analyses towards a more nuanced understanding of thermodynamic processes.
This research establishes a connection between the entropy of mixing and observational entropy, framing the former as a specific instance within an observer-dependent thermodynamic framework, intrinsically linking entropy and the potential for work extraction to the resolution of measurement. It moves beyond traditional analyses reliant on local thermal equilibrium, actively resolving the long-standing Gibbs mixing paradox by clarifying that knowledge of particle types does not translate to increased work extraction if those particles remain operationally indistinguishable through measurement. The work derives a Landauer-like principle, establishing an upper bound on the energy difference obtainable by two observers, introducing the concept of an ‘observational temperature’, a parameter determined by the accessible information within the system. This temperature effectively quantifies the thermodynamic cost associated with acquiring information and utilising it for work, moving beyond the limitations of conventional thermodynamic analyses, which typically assume equilibrium conditions.
This research provides a rigorous methodology for determining the energy required to unmix gases in non-equilibrium scenarios, extending beyond simple gas mixtures and offering a broader framework applicable to the thermodynamics of isolated classical gases generally. The findings actively contribute to a more nuanced understanding of entropy, work, and information in systems far from equilibrium, demonstrating that information plays a critical role in defining thermodynamic limits and energy transfer. Future work should investigate the practical limitations of measurement resolution and its impact on energy extraction; quantifying the energy cost associated with increasing measurement precision could provide valuable insights into the fundamental limits of thermodynamic processes. Furthermore, exploring the potential for utilising observational entropy as a resource for information processing and computation represents a potentially fruitful direction for future research.
👉 More information
🗞 Work and entropy of mixing in isolated quantum systems
🧠 DOI: https://doi.org/10.48550/arXiv.2507.05054