Researchers at the University of Bologna have made a remarkable achievement in nanotechnology by harnessing the power of light to create a molecular fit that was previously thought impossible. Led by Professor Alberto Credi, the team has successfully inserted a filiform molecule into the cavity of a ring-shaped molecule using a combination of photochemical reactions and self-assembly processes.
This innovative approach has the potential to pave the way for new methodologies in chemical synthesis and the development of dynamic molecular materials and devices. The study, published in the prestigious scientific journal Chem, was a collaboration between the University of Bologna, the University of Coruña in Spain, and the Isof-Cnr Institute in Bologna, with funding from the Ministry of University and Research.
Key researchers involved in the project include Neira, Chiara Taticchi, Federico Nicoli, and Massimiliano Curcio, among others. The breakthrough has implications for various areas of technology and medicine, and was developed at the Center for Light Activated Nanostructures, a joint laboratory between the University of Bologna and CNR.
Introduction to Light-Induced Molecular Self-Assembly
Nanotechnology has witnessed significant advancements in recent years, focusing on the self-assembly of molecular components to create systems and materials with structures on the nanometer scale. One of the fundamental processes in nanotechnology is the self-assembly of molecules, which takes advantage of the tendency of molecules to evolve toward a state of thermodynamic equilibrium characterized by minimum energy.
However, living organisms often function through chemical transformations that occur away from thermodynamic equilibrium, requiring external energy sources to drive these processes. Replicating such mechanisms with artificial systems is a complex and ambitious challenge, but one that could enable the creation of new substances capable of responding to stimuli and interacting with their environment.
The concept of molecular self-assembly has been explored extensively in recent years, with researchers seeking to develop new methodologies for chemical synthesis and the creation of dynamic molecular materials and devices. A team led by Prof. Alberto Credi of the University of Bologna has made a significant contribution to this field by demonstrating the use of light energy to drive the self-assembly of molecular components into a high-energy geometry that is not possible at thermodynamic equilibrium. This achievement has far-reaching implications for the development of new technologies and materials, particularly in areas such as medicine and nanotechnology.
The study published in the journal Chem highlights the potential of light-induced molecular self-assembly to create complex molecular structures that can be used to develop innovative materials and devices. The researchers employed a combination of photochemical reactions and self-assembly processes to insert a filiform molecule into the cavity of a ring-shaped molecule, resulting in a molecular “fit” that would otherwise be inaccessible. This breakthrough has the potential to pave the way for new methodologies of chemical synthesis and the development of dynamic molecular materials and devices that operate under non-equilibrium conditions, similar to living beings.
The use of light energy to drive molecular self-assembly offers several advantages, including the ability to control the formation of complex molecular structures and the potential to create dynamic systems that can respond to changes in their environment. The researchers’ findings have significant implications for the development of new materials and technologies, particularly in areas such as nanotechnology and medicine. Furthermore, the study demonstrates the importance of interdisciplinary research collaborations, bringing together experts from various fields to tackle complex challenges and drive innovation.
Mechanisms of Light-Induced Molecular Self-Assembly
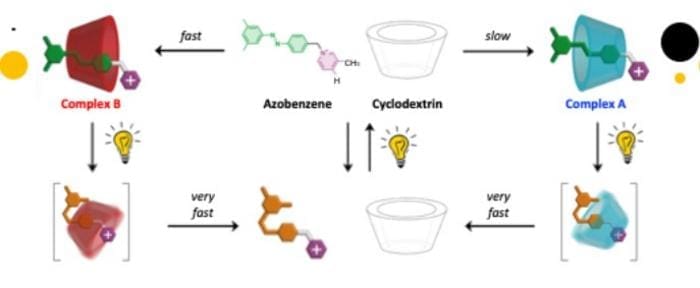
The mechanism of light-induced molecular self-assembly involves the use of photochemical reactions to drive the formation of complex molecular structures. In this study, the researchers employed a combination of photochemical reactions and self-assembly processes to insert a filiform molecule into the cavity of a ring-shaped molecule. The filiform compound possesses two different ends, and since the two rims of the cyclodextrin are also different, insertion of the former into the latter generates two distinct complexes, which differ in the relative orientation of the two components.
The formation of these complexes is influenced by the presence of light, which can convert the azobenzene from an extended configuration to a bent one, incompatible with the cavity. As a result, the complex dissociates, but the same light can convert the azobenzene back from the bent to the extended form, and the dissociated components can reassemble. Because complex B forms much faster than A, under continuous illumination, a steady state is reached in which complex B is the dominant product. Once the light is turned off, the azobenzene slowly reverts to the extended form, and after some time, only the A complex is observed.
This self-assembly mechanism coupled with a photochemical reaction makes it possible to harness the energy of light to accumulate unstable products, thus paving the way for new methodologies of chemical synthesis and the development of dynamic molecular materials and devices. The use of light-induced molecular self-assembly offers several advantages, including the ability to control the formation of complex molecular structures and the potential to create dynamic systems that can respond to changes in their environment.
Applications of Light-Induced Molecular Self-Assembly
The applications of light-induced molecular self-assembly are diverse and far-reaching, with potential uses in areas such as medicine, nanotechnology, and materials science. The development of new methodologies for chemical synthesis and the creation of dynamic molecular materials and devices could lead to significant advancements in these fields. For example, the use of light-induced molecular self-assembly could enable the creation of novel drug delivery systems, where the release of a therapeutic agent is triggered by changes in the molecular structure of the carrier.
Additionally, the development of dynamic molecular materials and devices that operate under non-equilibrium conditions could lead to significant advancements in areas such as nanotechnology and energy storage. The use of light-induced molecular self-assembly could also enable the creation of novel sensors and diagnostic tools, where changes in the molecular structure of the sensor are used to detect specific analytes or biomarkers.
The study published in Chem demonstrates the potential of light-induced molecular self-assembly to create complex molecular structures that can be used to develop innovative materials and devices. The researchers’ findings have significant implications for the development of new technologies and materials, particularly in areas such as medicine and nanotechnology. Furthermore, the study highlights the importance of interdisciplinary research collaborations, bringing together experts from various fields to tackle complex challenges and drive innovation.
In conclusion, the study published in Chem demonstrates the potential of light-induced molecular self-assembly to create complex molecular structures that can be used to develop innovative materials and devices. The use of photochemical reactions to drive the formation of complex molecular structures offers several advantages, including the ability to control the formation of complex molecular structures and the potential to create dynamic systems that can respond to changes in their environment.
The applications of light-induced molecular self-assembly are diverse and far-reaching, with potential uses in areas such as medicine, nanotechnology, and materials science. While there are several challenges that need to be addressed before this technology can be widely adopted, ongoing research is likely to lead to significant advancements in this field.
The study highlights the importance of interdisciplinary research collaborations, bringing together experts from various fields to tackle complex challenges and drive innovation. As research in this area continues to evolve, it is likely that we will see significant advancements in areas such as medicine, nanotechnology, and materials science, leading to the development of new technologies and materials that can improve our daily lives.
External Link: Click Here For More