Optical coherence microscopy currently struggles to image deep within tissues while maintaining the resolution needed to observe individual cells, hindering investigations of complex biological processes, but a new approach overcomes these limitations. Nobuhisa Tateno, Yue Zhu, Suzuyo Komeda, and colleagues at various institutions have developed a spatially coherent full-field optical coherence microscope that achieves cellular-resolution imaging at depths previously unattainable. The team’s innovative system incorporates refocusing techniques and a repetitive acquisition protocol, enabling not only detailed three-dimensional structural analysis, but also visualisation of dynamic processes occurring within tissues, demonstrated successfully on human breast cancer cell models. This breakthrough promises to significantly advance studies in developmental biology, cancer research, and other fields requiring detailed observation of living tissues.
Dynamic Imaging of Tissue and Spheroids
This research details advancements in dynamic optical coherence tomography (DOCT) and full-field swept-source optical coherence tomography (FF-SS-OCT), improving image quality and enabling the visualization of dynamic processes within biological tissues, such as tumor spheroids. Scientists are refining these techniques to study how tissues move and change over time, crucial for understanding drug responses. Full-field OCT offers high-speed, high-resolution imaging, and researchers address image distortions with computational refocusing to improve clarity. Understanding and mitigating speckle noise is also crucial for accurate imaging.
A major focus is reducing crosstalk in FF-SS-OCT, a common source of image artifacts, utilizing strategies like off-axis reference beams, multimode fiber, and advanced techniques for crosstalk-free imaging. Aberration correction, achieved through computational adaptive optics, further enhances image clarity. This technology allows scientists to visualize activity within tissues, and is being applied to study tumor spheroids, alveolar organoids, and evaluate drug responses. The signal strength in DOCT is affected by both the wavelength of light used and the desired resolution. Number fluctuation OCT allows for the measurement of flow velocity and other dynamic parameters within tissues, while sub-diffusion flow velocimetry provides a method for measuring slow flows. This research pushes the boundaries of optical coherence tomography, creating a powerful tool for studying dynamic biological processes with potential applications in drug discovery, personalized medicine, and fundamental biological research.
Deep Tissue Imaging via Coherent Microscopy
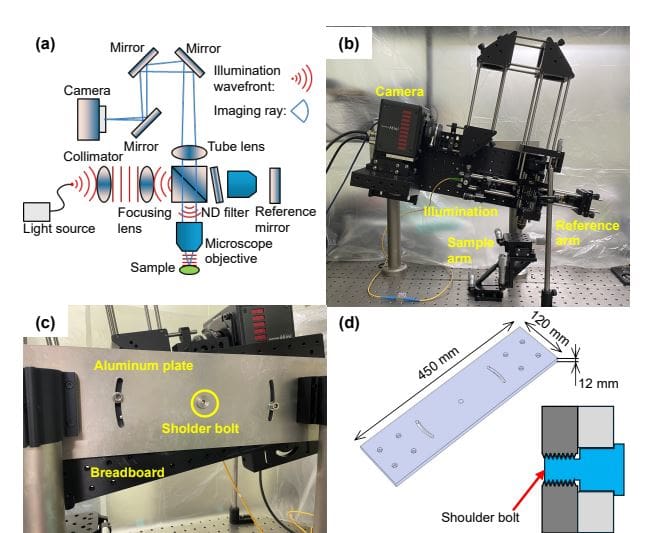
Scientists engineered a spatially coherent full-field optical coherence microscopy (SC-FFOCM) system with computational refocusing to overcome limitations in imaging thick biological samples, such as breast cancer cell spheroids. Recognizing that standard optical coherence tomography struggles to simultaneously achieve cellular-level resolution and deep imaging depth, they developed a system that utilizes flood illumination instead of point-scanning, eliminating energy loss and simplifying aberration correction. This innovative approach employs a high numerical aperture objective to deliver the necessary resolution while maintaining signal strength throughout the sample depth. To address surface reflections from the culture medium, the team designed a tilting mechanism for the entire optical system, allowing for precise adjustments of up to ±28 degrees to ensure optimal image clarity.
To minimize vibrations, the light source and computer were positioned separately from the optical table, and the camera fan was deactivated during image acquisition. Beyond structural imaging, the study pioneered a method for visualizing dynamic processes within the tissue, implementing a repetitive volume acquisition protocol to capture time-sequential data and reveal intra-tissue activities. The SC-FFOCM system achieves an in-focus lateral resolution of 1. 4 um and an axial resolution of 6. 5 um, allowing for volumetric dynamic imaging with cellular-level detail across the entire depth of the spheroid. This combination of high-resolution imaging and dynamic capability provides a powerful tool for studying complex biological processes within thick, three-dimensional tissue models.
Deep Tissue Imaging with Coherent Microscopy
Scientists have developed a spatially coherent full-field optical coherence microscopy (SC-FFOCM) system that overcomes limitations in imaging depth and enables visualization of dynamic processes within thick tissue samples. Traditional optical coherence tomography (OCT) struggles to simultaneously achieve high resolution and deep imaging, as increasing resolution typically reduces imaging depth. This research delivers a breakthrough by combining high-resolution imaging with the ability to see deeper into samples, specifically human breast adenocarcinoma spheroids. The team achieved a lateral resolution of 1.
4 um and an axial resolution of 6. 5 um in air, demonstrating the system’s capacity to resolve cellular-level details. Crucially, the SC-FFOCM incorporates computational refocusing, which corrects for the loss of focus that occurs when imaging deep within a sample, and avoids signal reduction common in standard point-scanning OCT. By utilizing a flood-illumination approach with a spatially coherent light source, the system eliminates the need for a confocal pinhole, preserving signal strength even at greater depths. Beyond static imaging, the researchers designed a repetitive volume acquisition protocol to visualize intra-tissue activities, a capability termed dynamic OCT (DOCT).
This involved acquiring 32 sequential OCT volumes, enabling the observation of dynamic processes within the spheroids. Synchronization of the wavelength-swept light source and a high-speed camera, operating at 4,000 frames per second, was essential for capturing these dynamic events. The system’s design incorporates a tilting mechanism to minimize surface reflections from the culture medium, and vibration isolation to ensure image stability. This combination of high-resolution imaging, computational refocusing, and dynamic acquisition delivers a powerful new tool for studying complex biological systems.
Volumetric Dynamic Imaging of Thick Samples
This research demonstrates a spatially coherent full-field optical coherence microscope, incorporating computational refocusing and dynamic optical coherence tomography, to achieve high-resolution imaging of thick samples. The newly developed system attains a lateral resolution of 1. 4 micrometers and an axial resolution of 6. 5 micrometers, enabling detailed visualization of three-dimensional structures and intra-tissue activities. Researchers successfully applied this imaging modality to human breast cancer cell spheroids, demonstrating volumetric dynamic imaging across the entire depth of the sample, a significant advancement over conventional methods.
Comparative studies reveal improved image penetration with this full-field technique compared to point-scanning optical coherence tomography, suggesting its potential for more comprehensive analysis of thick in vitro models. Researchers acknowledge that the acquisition time of the full-field system is longer than that of point-scanning methods, which may influence the observation of very rapid dynamic processes within the sample. These findings establish spatially coherent full-field optical coherence microscopy as a valuable tool for both structural and functional imaging, particularly for investigating complex biological samples and advancing in vitro research.
👉 More information
🗞 Dynamic full-field swept-source optical coherence microscope for cellular-resolution, long-depth, and intratissue-activity imaging
🧠 ArXiv: https://arxiv.org/abs/2511.10235