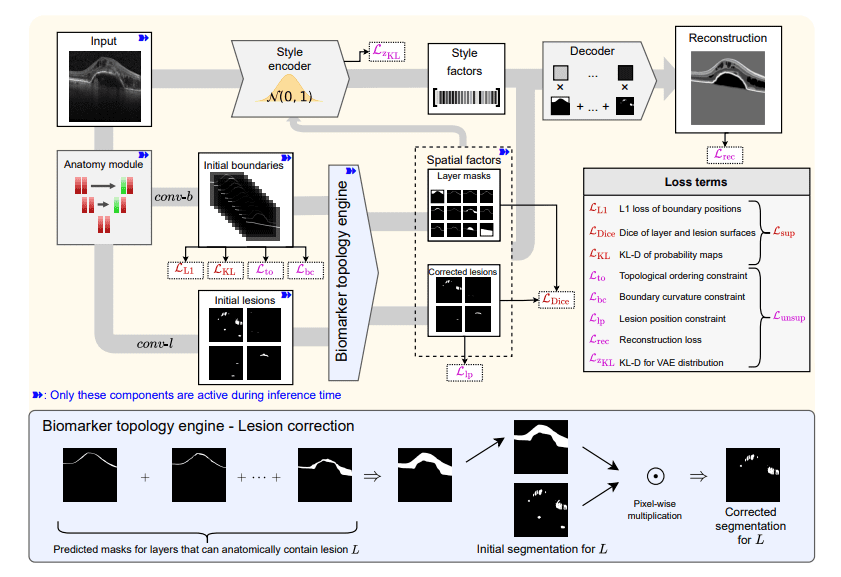
Optical coherence tomography (OCT) plays a crucial role in diagnosing and monitoring retinal diseases, and accurate segmentation of retinal layers and lesions is essential for effective patient care. Botond Fazekas, Guilherme Aresta, and Philipp Seeböck, working with colleagues at the Christian Doppler Laboratory for Artificial Intelligence in Retina and with Ursula Schmidt-Erfurth and Hrvoje Bogunović, present a new approach to this challenge. Their research addresses limitations in existing semi-supervised learning methods, which often produce anatomically unrealistic segmentations and struggle to model the complex relationship between layers and lesions. The team develops a novel model incorporating a fully differentiable ‘topology engine’ that enforces anatomical correctness, enabling joint learning and improved segmentation of both layers and lesions, even with limited labelled data. This advancement yields more realistic and robust biomarker segmentation, significantly outperforming current state-of-the-art methods on both public and internal datasets and demonstrating the potential for accurate, trustworthy retinal analysis.
Accurate segmentation is essential for diagnosing and monitoring retinal diseases like age-related macular degeneration and diabetic macular edema, but is complicated by the thinness of retinal layers, image noise, and variations between individuals. The team developed a novel deep learning framework to improve segmentation, particularly when limited labeled data is available. The framework utilizes a multi-part loss function, combining losses that focus on segmentation accuracy, precise boundary delineation, and consistency of predictions.
A key innovation, SoftAdapt, dynamically adjusts the weights of these loss functions during training, allowing the model to prioritize the most important aspects of the task at different stages. Furthermore, the framework incorporates semi-supervised learning techniques, effectively leveraging unlabeled data to enhance performance when labeled data is scarce. The team explored and utilized advanced network architectures, including U-Net, EfficientNet, Swin UNETR, and Mamba, for optimal feature extraction and segmentation. Evaluation on standard retinal OCT datasets demonstrates that the proposed framework achieves state-of-the-art results, significantly improving segmentation accuracy compared to existing methods.
The semi-supervised learning techniques effectively leverage unlabeled data, leading to substantial performance gains with limited labeled data. The SoftAdapt mechanism successfully adapts loss weights during training, improving performance and stability. The framework also demonstrates good generalizability to different datasets and imaging conditions.
Anatomically Consistent Retinal Biomarker Segmentation Model
This study presents a novel semi-supervised model for segmenting retinal biomarkers from optical coherence tomography (OCT) scans. Existing methods often produce anatomically implausible results and fail to accurately model the interplay between retinal layers and lesions. Researchers engineered a fully differentiable biomarker topology engine, a core innovation that enforces anatomically correct segmentation of both lesions and layers. This engine integrates prior anatomical knowledge directly into the segmentation process, constraining the possible locations of lesions based on the expected arrangement of retinal layers and vice versa.
The methodology employs a unique approach to learning, leveraging both labeled and unlabeled data to improve segmentation performance, particularly in pathological cases where manual annotation is scarce. Scientists developed a system that learns a disentangled representation, separating spatial factors, the location and shape of biomarkers, from style factors, variations in image appearance due to noise or individual differences. This separation enables more realistic layer segmentations and improves lesion detection, while simultaneously enforcing anatomically plausible positioning of lesions relative to the segmented layers. The team validated the approach on both public and internal datasets of OCT scans, demonstrating superior performance in both lesion and layer segmentation compared to current state-of-the-art techniques. The system achieves improved accuracy by explicitly modeling the bidirectional influence between layers and lesions, allowing for a more robust and reliable segmentation even in the presence of complex pathological features. This methodology demonstrates the potential of incorporating anatomical constraints into semi-supervised learning, paving the way for accurate, robust, and trustworthy retinal biomarker segmentation in clinical practice.
Retinal Layer and Lesion Segmentation with Topology
Scientists have developed a novel semi-supervised model for segmenting retinal biomarkers in optical coherence tomography (OCT) scans, achieving state-of-the-art performance in both layer and lesion segmentation. The core of this breakthrough lies in a fully differentiable biomarker topology engine, which enforces anatomically correct segmentation by modeling the complex interplay between retinal layers and lesions. This engine enables joint learning with bidirectional influence, meaning the model simultaneously considers how layers constrain lesion locations and how lesions deform layer morphology. The research team implemented a disentangled representation, crucial for effectively integrating unlabeled and partially annotated data, allowing the model to learn from diverse sources.
Extensive evaluation on both public and internal datasets demonstrates the model’s ability to generalise layer segmentation to pathological cases, a significant advancement over existing methods. This approach accurately models the intricate relationship between layers and lesions, ensuring topological consistency, a key challenge in automated retinal image analysis. By enforcing strict anatomical constraints, the model delivers more realistic layer segmentations and improves lesion segmentation, ensuring lesions are located in anatomically plausible positions relative to the segmented layers. This work demonstrates the potential of leveraging anatomical knowledge in semi-supervised learning for accurate, robust, and trustworthy retinal biomarker segmentation, paving the way for improved clinical diagnosis and monitoring of retinal diseases.
Retinal Layer and Lesion Segmentation Improved Significantly
This research presents a novel approach to segmenting retinal biomarkers in optical coherence tomography (OCT) scans, achieving significant improvements in both layer and lesion identification. The team developed a model that simultaneously segments retinal layers and lesions, incorporating topological constraints and anatomical priors to ensure anatomically plausible results. The model’s performance surpasses current state-of-the-art methods in both layer and lesion segmentation, demonstrating a statistically significant margin of improvement. Crucially, the research also demonstrates the model’s ability to generalise layer segmentation to pathological cases using partially annotated training data, a valuable capability for real-world clinical applications.
👉 More information
🗞 SD-RetinaNet: Topologically Constrained Semi-Supervised Retinal Lesion and Layer Segmentation in OCT
🧠 ArXiv: https://arxiv.org/abs/2509.20864