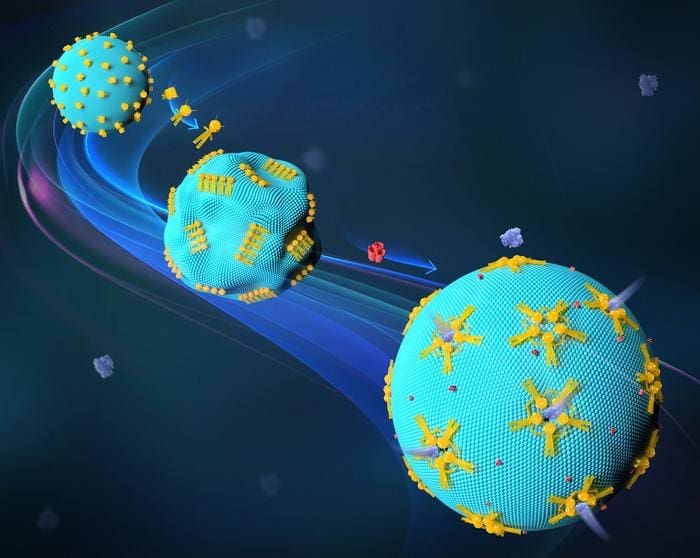
Scientists at the University of Stuttgart have made a crucial advancement in synthetic biology by developing reconfigurable DNA nanorobots that can alter artificial cells. Led by Professor Laura Na Liu, the team has successfully controlled the structure and function of biological membranes using DNA origami technology.
This innovation may facilitate the transportation of large therapeutic loads into cells, opening up new possibilities for targeted medication administration. The system developed by Professor Liu and her team, including Professors Stephan Nussberger and Hao Yan, allows for the creation of novel transport channels that can pass therapeutic proteins across cell membranes.
This breakthrough has the potential to revolutionize the field of synthetic biology and improve therapeutic strategies. The research was published in the journal Nature Materials, with contributions from the University of Stuttgart, the Max Planck Institute for Solid State Research, and Arizona State University’s Biodesign Center for Molecular Design and Biomimetics.
Introduction to DNA Nanorobots in Synthetic Biology
The field of synthetic biology has witnessed significant advancements with the development of DNA nanorobots that can alter artificial cells. Researchers at the University of Stuttgart, led by Prof. Laura Na Liu, have successfully controlled the structure and function of biological membranes using “DNA origami.” This innovative approach may facilitate the transportation of large therapeutic loads into cells, opening up new possibilities for targeted medication administration and other therapeutic interventions. The study, published in Nature Materials, highlights the potential of DNA nanotechnology in creating novel transport channels that can facilitate the passage of therapeutic proteins across cell membranes.
The concept of “form follows function” is a fundamental principle in modern design and architecture, which also applies to biological systems. The shape and morphology of a cell play a crucial role in its biological function. However, transferring this principle to artificial cells is a challenge in synthetic biology. Advances in DNA nanotechnology have provided promising solutions, enabling the creation of novel transport channels that can facilitate the passage of therapeutic proteins across cell membranes. Prof. Laura Na Liu and her team have developed an innovative tool for controlling the shape and permeability of lipid membranes in synthetic cells, which are made up of lipid bilayers that enclose an aqueous compartment.
DNA nanorobots have enabled programmable interactions with synthetic cells, allowing researchers to influence the shape and functionality of these cells. The team worked with giant unilamellar vesicles (GUVs), which are simple, cell-like structures that can be used as model systems for studying cellular processes. By using DNA origami structures as reconfigurable nanorobots, the researchers were able to reversibly change their shape and influence their immediate environment in the micrometer range. This transformation of DNA nanorobots was coupled with the deformation of GUVs and the formation of synthetic channels in the model GUV membranes.
Mechanisms of DNA Nanorobots in Synthetic Cells
The mechanism of DNA nanorobots on GUVs has no direct biological equivalent in living cells, making it an exciting area of research. The transformation of these DNA nanorobots can be coupled with the deformation of GUVs and the formation of synthetic channels in the model GUV membranes. These channels allowed large molecules to pass through the membrane and can be resealed if necessary. This means that researchers can use DNA nanorobots to design the shape and configuration of GUVs to enable the formation of transport channels in the membrane.
The study raises new questions about the potential of synthetic platforms, such as DNA nanorobots, to be designed with less complexity than their biological counterparts while still functioning in a biological environment. The system of cross-membrane channels created by DNA nanorobots allows for efficient passage of certain molecules and substances into cells. Most importantly, these channels are large and can be programmed to close when needed. When applied to living cells, this system can facilitate the transportation of therapeutic proteins or enzymes to their targets in the cell.
Applications of DNA Nanorobots in Therapeutic Strategies
The new study is an important step towards understanding disease mechanisms and improving therapies. The system of cross-membrane channels created by DNA nanorobots offers new possibilities for the administration of drugs and other therapeutic interventions. By facilitating the transportation of therapeutic proteins or enzymes to their targets in the cell, this system can improve the efficacy of treatments. Prof. Hao Yan, one of the co-authors of the study, notes that the approach opens up new possibilities to mimic the behavior of living cells, which could be crucial for future therapeutic strategies.
The interdisciplinary research team, including members from several institutes at the University of Stuttgart, has made significant contributions to the field of synthetic biology. The study demonstrates the potential of DNA nanotechnology in creating novel transport channels that can facilitate the passage of therapeutic proteins across cell membranes. As researchers continue to explore the possibilities of DNA nanorobots, we may see new breakthroughs in the development of targeted therapies and improved treatment outcomes.
Future Directions and Implications
The study highlights the potential of DNA nanorobots in synthetic biology and raises important questions about the future of therapeutic strategies. As researchers continue to develop and refine this technology, we may see new applications in fields such as medicine, biotechnology, and materials science. The ability to design and control the shape and configuration of GUVs using DNA nanorobots could lead to significant advances in our understanding of cellular processes and disease mechanisms.
The use of DNA nanorobots in synthetic cells also raises important questions about the potential for synthetic platforms to be designed with less complexity than their biological counterparts. As researchers continue to explore this area, we may see new breakthroughs in the development of novel therapeutic strategies and improved treatment outcomes. The study demonstrates the power of interdisciplinary research and collaboration in advancing our understanding of complex biological systems and developing innovative solutions to pressing medical challenges.
External Link: Click Here For More