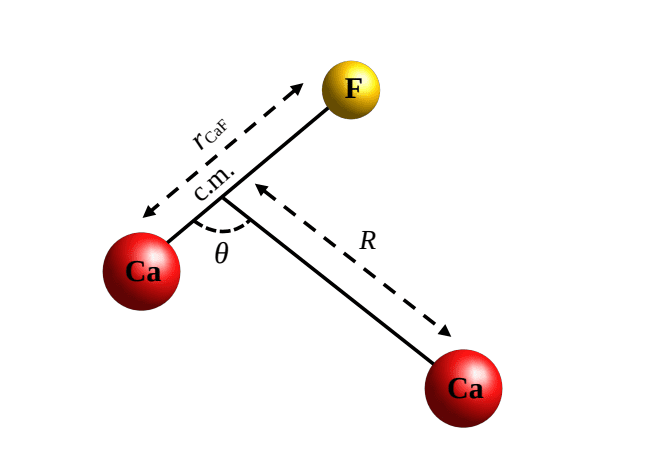
The subtle interactions between atoms underpin a vast range of chemical processes, and understanding these forces is crucial for controlling reactions at the quantum level. Dibyendu Sardar from the University of Warsaw and John L. Bohn from JILA, NIST, and the University of Colorado Boulder, alongside their colleagues, investigate the interactions between laser-cooled calcium fluoride and calcium atoms, exploring the potential for atom exchange. Their work computes detailed potential energy surfaces for various electronic states, revealing a surprisingly deep and anisotropic ground state, and importantly, an excited state that lies below the energy required to form the molecule. These findings illuminate the pathways for atom exchange reactions, offering new insights into controlling chemical interactions with precision and potentially enabling novel approaches to ultracold chemistry.
The research focuses on calculating potential energy surfaces that govern these interactions, providing insights into the dynamics of collisions between these species. Calculations employ sophisticated quantum chemical methods to determine the electronic structure of the system, accurately accounting for relativistic effects and core polarization, allowing for precise determination of the potential energy as a function of interatomic separation and revealing key features such as stable complexes and barriers that control reaction rates. The calculated potential energy surfaces demonstrate the existence of long-range interactions dominated by dipole-dipole and dispersion forces, alongside shorter-range covalent contributions arising from orbital overlap. These surfaces provide a foundation for understanding the mechanisms of atom exchange and for predicting the rates of these reactions at ultralow temperatures. The study also explores how isotopic substitution influences the potential energy surfaces, revealing subtle shifts in the energy landscape and their impact on reaction dynamics.
Ultracold Calcium Fluoride Collisions and Interactions
This research details ultracold calcium fluoride molecules, their interactions with other atoms like rubidium, sodium, and strontium, and the theoretical calculations used to understand these interactions. A significant focus is on the interactions between calcium fluoride and rubidium atoms, including the potential for forming ultracold molecules through photoassociation. Researchers utilize databases containing spectroscopic data for calcium fluoride and employ techniques like photoassociation and Feshbach resonances to understand these interactions. Sophisticated quantum chemistry calculations model the potential energy surfaces for these interactions, using density functional theory, coupled cluster methods, Gaussian basis sets, and pseudopotentials to ensure accuracy. The creation of accurate potential energy surfaces is crucial for simulating collision dynamics, and calculations of molecular properties like dipole polarizabilities are essential for understanding interactions between molecules and external fields. This research represents a comprehensive effort to understand the fundamental interactions of ultracold calcium fluoride molecules, with the ultimate goal of creating and controlling these molecules for applications in quantum science and technology.
Excited States Drive Calcium Fluoride Reactions
This research presents detailed investigations into the interactions between laser-cooled calcium fluoride and calcium atoms, aiming to understand the pathways of chemical reactions between these species. Through advanced theoretical calculations, scientists have mapped the potential energy surfaces for multiple electronic states of the resulting trimer molecule, revealing strongly bound configurations and identifying key features that govern atom exchange processes. Notably, the calculations demonstrate that certain excited electronic states possess lower energy than the ground state, suggesting these states play a significant role in the reaction dynamics. The team computed two-dimensional potential energy surfaces for both the ground and excited states, revealing a complex landscape with both global and local minima accessible at ultracold collision energies, indicating stable configurations for the trimer molecule.
Analysis of the potential energy surfaces also revealed strong anisotropy in the interactions, with the shape of the surface significantly influencing the dynamics of the reaction. Researchers determined important coefficients that describe the long-range interactions between calcium fluoride and calcium, providing valuable parameters for studying ultracold collisions. The authors acknowledge that their calculations employed the rigid rotor approximation for the calcium fluoride molecule, which may introduce some limitations, and suggest future work could explore the effects of molecular vibrations on the potential energy surfaces and reaction pathways. These detailed theoretical results provide a foundation for future experimental studies of ultracold collisions and chemical reactions involving calcium fluoride and calcium, potentially leading to new insights into controlling chemical processes at extremely low temperatures.
👉 More information
🗞 Ground and excited potential energy surfaces for CaF+Ca interactions and isotope exchange reactions
🧠 ArXiv: https://arxiv.org/abs/2510.23303