Wentian Zheng of Peking University and collaborators have successfully integrated quantum sensing with scanning probe microscopy (SPM) to create an instrument capable of triggering and observing chemical reactions at nanometer resolution. This novel approach, detailed in Physics, monitored the elementary steps of water dissociation at a solid-liquid interface, utilizing a combined technique to locally induce and detect the reaction P + n e– ⇔ Q. By overcoming limitations of existing surface analysis methods – such as spatially averaged signals from nonlinear spectroscopies and the inability to detect unpaired electrons with SPM – this advancement offers a comprehensive view of nanoscale chemical processes critical to fields including chemistry, biology, and earth science.
Quantum Sensing and SPM Convergence
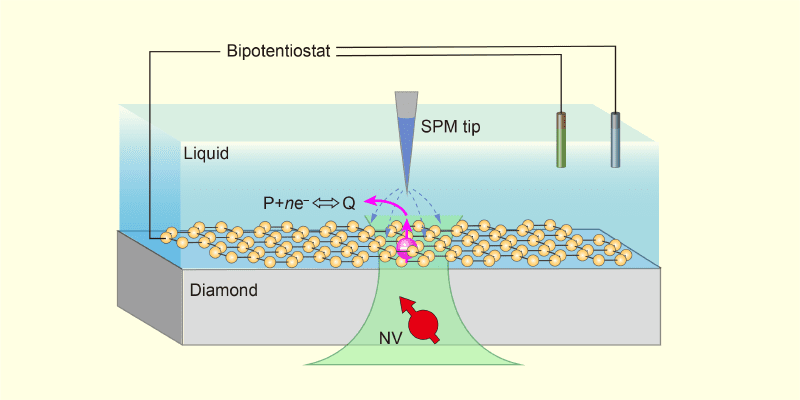
Researchers have converged quantum sensing with scanning probe microscopy (SPM) to create a novel instrument, NV-SPM, capable of triggering and observing chemical reactions at the nanoscale. This breakthrough utilizes nitrogen-vacancy (NV) centers in diamond as built-in electron spin resonance (ESR) or nuclear magnetic resonance (NMR) sensors. By employing an atomically sharp, electrically biased SPM tip positioned above a shallow NV center, scientists can inject electrons into interfacial water and initiate reactions – like water dissociation – while simultaneously monitoring the resulting electron and proton dynamics with nanometer resolution.
The NV-SPM relies on the qPlus force sensor for stable operation, even in liquid environments. In a recent demonstration, researchers successfully triggered water dissociation by injecting electrons, observing the formation of hydrated electrons, hydroxide ions, and ultimately hydrogen peroxide. Crucially, the NV center detected intermediate species – like unpaired electrons in hydroxyl radicals – which are inaccessible to traditional SPM techniques. Monitoring proton lifetimes within the NV center’s detection volume (nanometers) further characterized proton diffusion at the interface.
Beyond the diamond-water interface explored in this study, the NV-SPM architecture is adaptable. Surfaces can be modified with electrodes for electrochemical investigations or coated with 2D materials to explore diverse solid-liquid reactions. This combined approach promises a new level of insight into interfacial chemistry, moving beyond inference to direct observation of molecular details – potentially revolutionizing fields from materials science to biology.
Monitoring Water Dissociation at Interfaces
Researchers have combined quantum sensing with scanning probe microscopy (SPM) to create NV-SPM—a tool capable of triggering and observing chemical reactions at the nanoscale. This breakthrough utilizes nitrogen-vacancy (NV) centers in diamond as built-in electron spin resonance (ESR) sensors. By applying a voltage to a sharp SPM tip positioned above the NV center, researchers initiated water dissociation at a solid-liquid interface. This allows direct observation of intermediate species—like hydrated electrons and hydroxyl radicals—critical in understanding interfacial chemistry.
The key to NV-SPM’s power lies in its ability to detect unpaired electrons. Traditional surface analysis techniques often average signals over larger areas or cannot detect these crucial reaction intermediates. Zheng and colleagues demonstrated this by monitoring the formation of hydroxide ions and protons resulting from water dissociation. Moreover, they characterized proton diffusion within a nanometer-scale volume surrounding the NV center, providing insights into reaction dynamics previously inaccessible.
Beyond the diamond-water interface, NV-SPM holds promise for studying a variety of solid-liquid chemical reactions. By modifying the diamond surface with different materials—like evaporated electrodes or 2D materials—the technique can be adapted to investigate electrochemical reactions and various interfacial processes. This combined approach—local triggering and chemical identification—represents a significant step toward directly observing the molecular details of interfacial reactions.
NV Centers: Principles and Capabilities
Nitrogen-vacancy (NV) centers in diamond are emerging as powerful nanoscale sensors due to their unique quantum properties. These defects—a nitrogen atom adjacent to a missing diamond atom—exhibit photoluminescence sensitive to their spin state. This sensitivity allows detection of external stimuli like electric and magnetic fields, effectively functioning as nanoscale ESR or NMR sensors. Crucially, NV centers can identify chemical species at interfaces, offering a pathway to directly observe reactions – a capability lacking in many traditional surface analysis techniques.
The breakthrough enabling direct observation comes from combining NV center sensing with scanning probe microscopy (SPM) into a single instrument – NV-SPM. Utilizing a qPlus force sensor for stable operation, the SPM delivers a localized electric field to the diamond surface. This field extracts electrons, initiating chemical reactions at the NV center’s sensing location. In recent experiments, this setup triggered water dissociation, revealing key intermediates like hydrated electrons and hydroxyl radicals – species difficult to detect with conventional methods.
This NV-SPM technique isn’t limited to diamond-water interfaces. The diamond substrate can be modified with electrodes or 2D materials, extending its applicability to diverse solid-liquid systems. By injecting electrons with the SPM tip and then using nanoscale ESR/NMR from the NV center, researchers can analyze electron transfer, identify reaction products, and study diffusion dynamics – all with nanometer resolution. This combination promises a new era of direct observation in interfacial chemistry.
Applications and Future Potential
This new NV-SPM technique combines quantum sensing with scanning probe microscopy, enabling researchers to both trigger and observe chemical reactions at the nanoscale. Specifically, the team demonstrated water dissociation at a diamond-water interface by injecting electrons via a biased tip. Crucially, the nitrogen-vacancy (NV) center in diamond acted as a built-in ESR/NMR sensor, detecting unpaired electrons forming during the reaction – something traditional SPM methods cannot achieve. This localized control and chemical identification represents a significant advancement in interfacial chemistry.
The power of NV-SPM extends beyond simply detecting reaction intermediates. By monitoring the lifetime of proton signals within the NV center’s nanometer-scale detection volume, researchers were able to characterize proton diffusion at the interface. This opens doors to studying dynamic processes, not just static snapshots of reactions. Furthermore, while the initial demonstration used diamond, the technique is adaptable. Surfaces can be modified with 2D materials or electrodes to investigate a wide range of solid-liquid interfaces and electrochemical reactions.
This combined approach holds immense potential across numerous scientific disciplines. From understanding corrosion and catalysis to designing more efficient energy storage solutions, the ability to visualize and analyze interfacial reactions with both spatial and chemical specificity is transformative. Though challenges remain in applying this to complex systems, the proof-of-principle demonstrated by Zheng and colleagues paves the way for direct observation of molecular-level processes previously inferred through indirect methods.