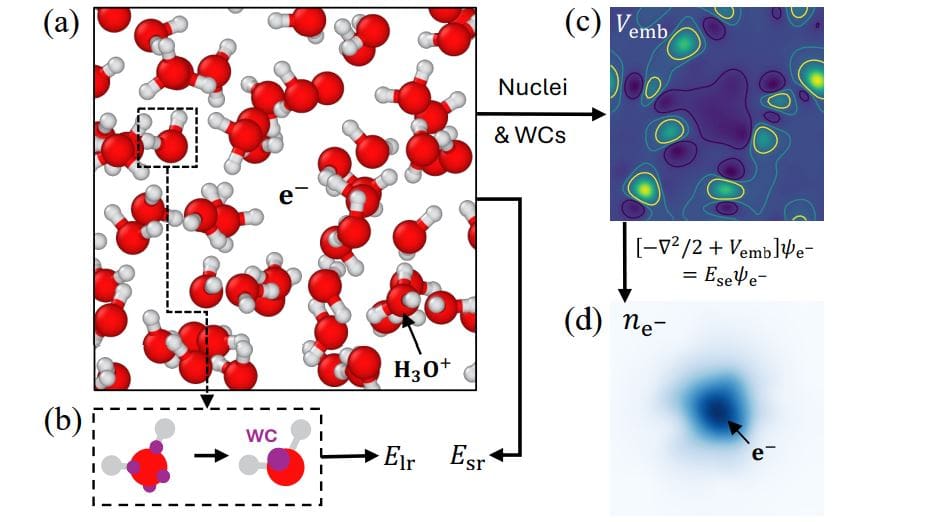
Understanding the behaviour of electrons in liquids presents a significant challenge for computational chemistry, as traditional methods struggle to accurately model these particles without excessive computational cost. Ruiqi Gao of Princeton University, Pinchen Xie from Lawrence Berkeley National Laboratory, and Roberto Car of Princeton University, along with their colleagues, have developed a new machine learning approach that directly incorporates the mechanical behaviour of an excess electron into molecular simulations. This innovative method achieves accuracy comparable to complex quantum calculations, but at a fraction of the computational expense, allowing researchers to study the interactions of solvated electrons in detail. The team successfully applied this technique to model the reaction of an electron with a hydronium ion in water, identifying a specific proton transfer mechanism and accurately predicting reaction rates and free energies that align closely with experimental observations, offering new insights into fundamental chemical processes.
Density functional theory calculations offer a computationally efficient approach to achieving accurate results, but struggle to explicitly model electrons. Scientists have developed an electron-aware machine-learning force field that addresses this limitation by treating an excess electron quantum mechanically, while employing machine learning to reproduce density functional theory calculations for the remaining interactions. This method accurately models the behavior of electrons in water and their reaction with hydronium ions, revealing a proton transfer mechanism where a proton recombines with an electron. The team determined forward reaction rates between 350 K and 450 K, demonstrating an Arrhenius relationship with an activation energy consistent with existing experimental measurements.
QM/ML Force Field Development and Validation
This research details the methodology used to investigate the reaction between an electron and a hydronium ion, forming a neutral hydrogen atom. The study focuses on accurately calculating reaction rates and determining equilibrium constants, employing a combined quantum mechanical/machine learning (QM/ML) force field. This innovative approach combines the accuracy of quantum mechanical calculations with the efficiency of machine learning, addressing the challenges of simulating this reaction accurately. The authors carefully validated their methods, demonstrating the reliability of their results.
The research involved detailed calculations of reaction rates using a survival probability method, emphasizing the importance of sampling from the Quasi-Stationary Distribution to avoid biased estimates. Finite-size scaling analysis was performed to extrapolate rate constants to the dilute limit, ensuring accuracy. Equilibrium constants were calculated using potential of mean force calculations and free energy integration, enhanced by a collective variable to improve sampling efficiency. Convergence of the potential of mean force was demonstrated with increasing system size, confirming the robustness of the calculations.
The team investigated the energy and spatial spread of the excess electron, correlating their modeled results with Kohn-Sham calculations from density functional theory. Differences between the modeled and DFT results were attributed to the absence of self-interaction in the model, highlighting areas for future improvement. Detailed analysis of the log-survival probability method was provided, showcasing the obtained results over time. The authors critically assessed the limitations of standard neural network potentials for this reaction, explaining their instability and inability to accurately capture the dynamics. They argue for the necessity of a more physical treatment of the excess electron, justifying their QM/ML approach. The development of the QM/ML force field, the use of the collective variable, finite-size scaling analysis, and addressing self-interaction error represent key technical innovations.
Hydronium Ion Reaction Mechanism Confirmed by Model
Scientists have developed a new method to model the behavior of electrons in water and their reaction with hydronium ions, achieving both accuracy and efficiency through a physics-based approach. Simulations revealed a proton transfer mechanism governing the recombination of the excess electron with a proton, and determined reaction rates and equilibrium constants across a range of temperatures. The calculated activation energy aligns with existing experimental measurements, confirming the model’s accuracy. Comparisons with previous studies at 400 K further validate the results. The team also determined the equilibrium constant for the reaction, allowing calculation of the reaction free energy, which also aligns with experimental data.
Enhanced sampling simulations were employed to map the potential of mean force, crucial for determining the equilibrium constant. Results showed a van’t Hoff plot of pK1 decreasing with increasing temperature, indicating an endothermic reaction and mirroring experimental trends. The calculated reaction free energy at 350 K corresponds closely to experimental values. Diffusion coefficients were measured for water, the solvated electron, and the hydronium ion, yielding values that demonstrate reasonable agreement with established data. The research underscores the dramatic stabilizing effect of the solvent environment, as the reaction in a vacuum would require significantly more energy than observed in water. The developed model is extensible and applicable to a broad range of solvated-electron reactions, potentially advancing understanding of nonadiabatic electron transfer processes and bound excitons.
Electron Transfer in Water Modeled Accurately
Researchers have developed a new machine-learning method to model the behavior of electrons in water and their subsequent reactions with hydronium ions. This approach accurately represents the excess electron mechanically, while simultaneously reproducing calculations derived from density functional theory. Through simulations, the team identified a proton transfer mechanism governing the recombination of the excess electron with a proton, and determined reaction rates and equilibrium constants across a range of temperatures, achieving results consistent with experimental data. The developed model demonstrates accuracy, robustness, and efficiency, and is designed to be readily extended to other systems. The researchers acknowledge limitations in the underlying electronic structure methods and the neglect of nuclear quantum effects, suggesting these areas as priorities for future work. They also anticipate the applicability of their method to a wider range of reactions involving solvated electrons, including those involving nonadiabatic electron transfer, small polarons, and bound excitons, opening avenues for further investigation into these complex phenomena.
👉 More information
🗞 A Machine Learning Model for the Chemistry of a Solvated Electron
🧠 ArXiv: https://arxiv.org/abs/2511.22642