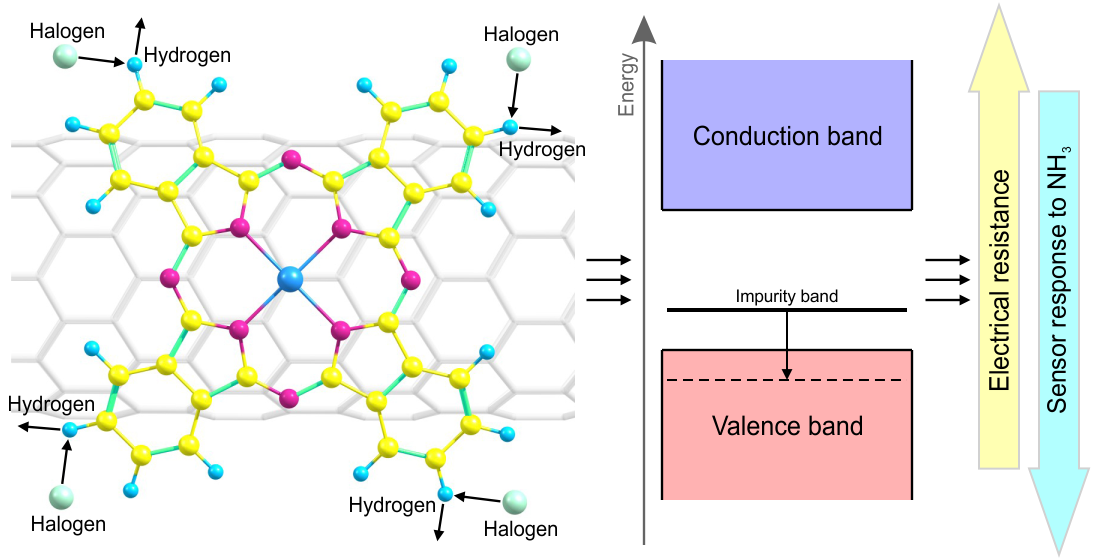
Hybrid materials, a class of materials that combine the properties of two or more different substances, have gained attention in recent years due to their potential applications in sensing technologies. Researchers have discovered that combining carbon nanotubes (CNTs) with tetra- or octa-halogen-substituted zinc phthalocyanines (ZnPc) creates a hybrid material that exhibits unique electrical conductivity properties.
This breakthrough has significant implications for developing advanced sensing technologies, particularly for detecting ammonia molecules. By using quantum-chemical modeling, researchers have gained valuable insights into these hybrids’ electronic structure and properties, paving the way for creating sensitive sensors that can detect even small concentrations of ammonia gas.
What are hybrid materials, and why are they important?
In this context, hybrid materials refer to a class of materials that combine the properties of two or more different substances. In this study, hybrid materials comprise carbon nanotubes (CNTs) and zinc phthalocyanines (ZnPcs). Carbon nanotubes are known for their exceptional electrical conductivity, mechanical strength, and thermal stability, while zinc phthalocyanines have unique optical and electronic properties. The combination of these two substances creates a material with potentially improved properties.
Hybrid materials are essential because of their potential applications in various fields, such as electronics, energy storage, and sensing technologies. This study explores the use of hybrid materials in ammonia sensors. Ammonia is an essential chemical compound in various industries, including agriculture, pharmaceuticals, and energy production. Accurate detection and monitoring of ammonia levels ensure safe working conditions and prevent environmental pollution.
The researchers involved in this study aim to understand the properties of hybrid materials based on CNTs and ZnPcs from a quantum-chemical point of view. They use computational methods to model the behavior of these materials at the atomic level, allowing them to predict their electrical conductivity and sensor response to ammonia molecules.
What is Quantum Chemical Modeling, and How Does it Relate to Hybrid Materials?
Quantum chemical modeling is a computational method for studying materials’ electronic structure and properties. In this context, researchers use the Density Functional-Based Tight Binding (DFTB) method to investigate the change in the band structure of hybrid materials based on CNTs and ZnPcs upon the adsorption of ammonia molecules.
The DFTB method is a simplified version of the density functional theory (DFT) method widely used in quantum chemistry. It is beneficial for studying large systems, such as hybrid materials, where the number of atoms can be in the thousands or even millions.
Using the DFTB method, researchers can calculate the electronic structure of hybrid materials and predict their electrical conductivity. This information is crucial for understanding how these materials respond to different chemical environments, such as the presence of ammonia molecules.
The study also employs the nonequilibrium Greens functions (NEGF) method to investigate the change in hybrid materials’ electrical conductivity upon the adsorption of ammonia molecules. The NEGF method is a powerful tool for studying the electronic transport properties of materials and can provide valuable insights into their behavior under different conditions.
What are the Key Findings of this Study?
The study presents several key findings related to the sensor response of hybrid materials based on CNTs and ZnPcs towards ammonia molecules. The researchers found that the electrical conductivity of these materials and their changes in the case of interaction with ammonia molecules depends on the position of the impurity band formed by the orbitals of macrocycle atoms relative to the forbidden energy gap of the hybrids.
The study also showed that the sensor response of the hybrids containing halogenated phthalocyanines was lower by one or two orders of magnitude compared to the hybrid with unsubstituted zinc phthalocyanine. This result was obtained by NEGF calculations, which demonstrated a change in the hybrids’ electrical conductivity and ammonia molecules’ adsorption.
The analysis revealed that to improve the sensor characteristics of CNT-based hybrid materials, preference should be given to those phthalocyanines in which substituents contribute to an increase in HOMO energy relative to the unsubstituted macrocycles. This finding has important implications for designing and developing new hybrid materials with improved sensing properties.
What are the Implications of this Study?
This study’s findings have significant implications for developing new hybrid materials with improved sensing properties. The researchers’ analysis suggests that by carefully designing the substituents on the phthalocyanine macrocycle, hybrid materials with enhanced sensor responses to ammonia molecules may be possible.
This study also highlights the importance of quantum chemical modeling in understanding the behavior of hybrid materials at the atomic level. By using computational methods such as DFTB and NEGF, researchers can gain valuable insights into these materials’ electronic structure and properties, allowing them to predict their behavior under different conditions.
Developing new hybrid materials with improved sensing properties has important implications for various industries, including agriculture, pharmaceuticals, and energy production. Accurate detection and monitoring of chemical compounds such as ammonia are crucial for ensuring safe working conditions and preventing environmental pollution.
Publication details: “Hybrid Materials Based on Carbon Nanotubes and Tetra- and Octa-Halogen-Substituted Zinc Phthalocyanines: Sensor Response Toward Ammonia from the Quantum-Chemical Point of View”
Publication Date: 2024-12-30
Authors: Павел О. Краснов, V. N. Ivanova, Darya Klyamer, Dmitry Bonegardt, et al.
Source: Sensors
DOI: https://doi.org/10.3390/s25010149