Controlling how molecules break apart remains a central challenge in molecular physics, and scientists are increasingly turning to nanoscale systems to achieve this control. Johan F. Triana from Universidad Católica del Norte and Felipe Herrera from Universidad de Santiago de Chile, along with their colleagues, now demonstrate a dramatic enhancement in the efficiency of breaking molecular bonds using infrared light trapped within a tiny cavity. Their research reveals that a molecule, specifically carbon monosulfide, requires significantly less energy to dissociate when positioned inside this cavity, achieving a reduction of two orders of magnitude in the laser energy needed. This enhancement stems from a purely mechanical effect, where the cavity fundamentally alters the way the molecule absorbs light and ultimately breaks apart, offering a new pathway for understanding and controlling chemical reactions at the single-molecule level and paving the way for innovative nanophotonic experiments.
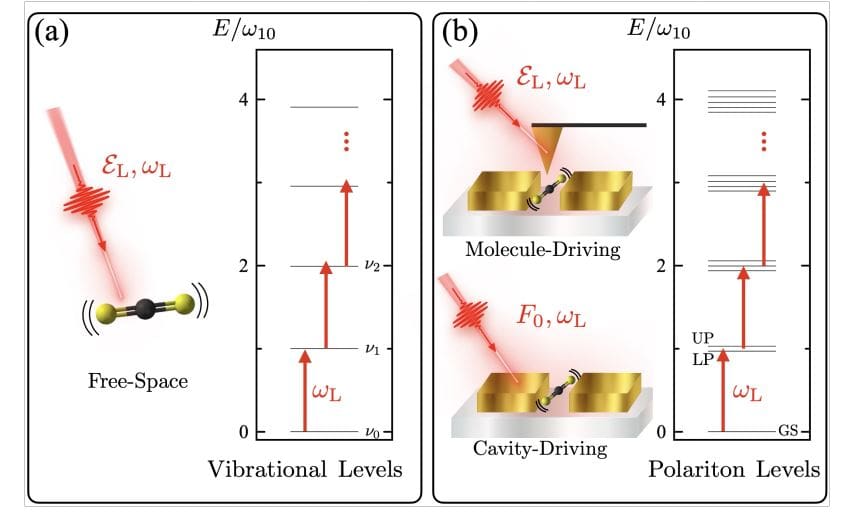
Currently, systems are being developed to explore unusual interactions between light and molecules within cavity quantum electrodynamics, but how chemical reactions proceed in these environments remains unclear. To address this, researchers study the infrared laser-induced breakdown of a single carbon disulphide molecule, focusing on a vibrational mode strongly coupled to the light within a resonant cavity. The results demonstrate that significantly less energy is required to break the molecule’s bonds inside the cavity compared to free space, with the efficiency depending on how the light interacts with the molecule.
Cavity Enhanced Spectroscopy of CS2 Dissociation
This supplementary information provides detailed theoretical and computational support for the research findings, explaining how the results were obtained and validating the methods used. It offers a comprehensive account of the calculations and approximations employed, ensuring reproducibility and critical evaluation. The appendices detail the quantum dynamics simulations and energy calculations. Appendix A focuses on quantum dynamics simulations using a mean-field approximation, a simplification technique used to model the interaction between the molecule and the light within the cavity. This approach allows for tractable calculations while maintaining reasonable accuracy.
Appendix B details the calculation of driving energy, the energy transferred from the laser to the molecule, in both direct and cavity-driven scenarios. This provides a clear definition of the energy driving the bond-breaking process. Appendix C compares the results obtained using a full quantum electrodynamics formulation with those from a simpler model, demonstrating the robustness of the findings. Appendix D presents dissociation probabilities as a function of the light-matter coupling strength, revealing that the activation time for bond breaking is directly related to how strongly the molecule interacts with the light. Overall, this supplementary information provides a rigorous and transparent account of the theoretical framework underpinning the research.
Enhanced Photodissociation Within Infrared Nanocavities
Scientists have achieved a significant breakthrough in understanding how molecules behave within infrared nanocavities, demonstrating remarkably efficient photodissociation. This work focuses on carbon disulphide molecules strongly coupled to the electromagnetic field inside a nanocavity, revealing a pathway to break chemical bonds with substantially less energy than in free space. Experiments demonstrate that photodissociation occurs with two orders of magnitude less laser energy when photons are directly injected into the cavity, compared to directly exciting the molecule’s vibrations. This enhancement is not simply due to increased laser power, but a purely quantum mechanical effect stemming from the unique interaction between the molecule and the cavity vacuum.
Researchers modeled the system using a Hamiltonian that accounts for the molecule’s vibrational energy, the cavity’s electromagnetic field, and their interaction, allowing for detailed analysis of the energy transfer process. The team observed that the cavity modifies the vibrational ladder climbing process, creating a high density of energy levels that facilitate bond breaking. Specifically, with a light-matter coupling strength of 0. 02, a driving energy of 0. 0025 aJ is sufficient to initiate significant dissociation when injecting photons directly into the cavity, compared to 0.
055 aJ required for comparable results when driving the molecule directly. These findings demonstrate that the nanocavity acts as a surrogate molecular mode, strongly interacting with the dissociative vibrations and dramatically lowering the energy barrier for bond breaking. This breakthrough provides a fundamental mechanistic understanding of chemical dynamics and opens new avenues for designing nanophotonic experiments that probe single-molecule interactions.
Nanocavities Boost Photodissociation Efficiency Dramatically
This research demonstrates a significant enhancement in the efficiency of molecular photodissociation when performed within infrared nanocavities, revealing fundamentally new dynamics compared to free-space environments. Scientists successfully show that breaking chemical bonds in a single carbon disulphide molecule requires substantially less laser energy inside a nanocavity, with the method proving particularly effective when photons are directly injected into the cavity itself. This improvement stems from a purely quantum mechanical effect where the cavity’s vacuum energy assists in the vibrational ladder-climbing process necessary for bond dissociation, effectively acting as a surrogate molecular mode. The team’s findings indicate that the cavity modifies the usual molecular dynamics, mixing multiple vibrational states and facilitating bond breaking through this vacuum-assisted mechanism. Researchers acknowledge that their current theoretical treatment relies on approximations, and future work will focus on incorporating more accurate descriptions of the nanocavity’s photonic modes, including factors like photon loss and dispersion. Nevertheless, this work establishes a pathway towards designing novel chemical control strategies that leverage quantum effects to minimize energy consumption in photodissociation processes and provides new insights into how photonic degrees of freedom influence molecular dynamics at the point of bond breaking.
👉 More information
🗞 Enhancing Infrared Laser Dissociation of Molecules with the Electromagnetic Vacuum
🧠 ArXiv: https://arxiv.org/abs/2511.17278