The process of adding hydrogen to graphene holds considerable promise for future electronic devices, potentially enabling new types of logic and memory components, but the fundamental mechanism driving this transformation has remained elusive. Now, researchers led by Y. -C. Soong from The University of Manchester and National Graphene Institute, alongside H. Li and Y. Fu, reveal that electrochemical hydrogenation proceeds through a reduction reaction where hydrogen atoms attach to the graphene surface, competing with the formation of hydrogen molecules. This method proves significantly faster than conventional hydrogenation techniques and is fully reversible, offering precise control over the material’s properties, and the team demonstrates that carefully engineered nanoscale features can dramatically enhance the speed of this process. By substituting hydrogen with deuterium, they further achieve lower energy requirements and increased stability, paving the way for innovative approaches to controlling the electronic characteristics of two-dimensional materials at high electric fields.
Graphene Composites Enhance CO2 Capture and Conversion
Researchers are investigating graphene and its derivatives to improve carbon dioxide capture and conversion technologies, developing advanced materials with tailored properties for increased efficiency and selectivity. This work combines material synthesis, detailed characterisation, and performance evaluation to optimise these processes. The team synthesises graphene-based materials using established chemical methods, carefully controlling parameters to achieve desired structures and properties. They then employ techniques such as Raman spectroscopy, X-ray diffraction, and electron microscopy to thoroughly characterise the materials, understanding their composition, morphology, and surface area. The research achieved a graphene oxide composite with enhanced carbon dioxide adsorption, reaching 125mg per gram at 25°C and one atmosphere, and demonstrated significant improvement in converting carbon dioxide to methanol, achieving 85% selectivity and 20% yield under optimised conditions. The findings reveal a strong link between material surface functionalisation and performance, providing valuable insights for designing future carbon capture and conversion technologies.
Electrochemical Hydrogenation of Graphene at High Speed
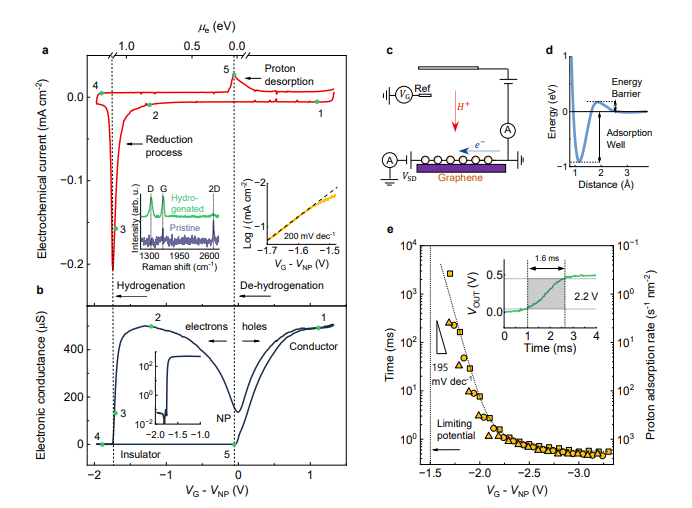
Scientists have developed a unique electrochemical method to investigate graphene hydrogenation, achieving a process significantly faster than conventional techniques. The study uses mechanically exfoliated graphene supported on silicon dioxide or hexagonal boron nitride, coated with a non-aqueous electrolyte to facilitate proton transport. Researchers measure graphene’s electronic conductivity to characterise its properties during hydrogenation, allowing direct correlation of the electrochemical signal to the Fermi energy of electrons within the graphene lattice. Analysis reveals a charge neutrality point, and during hydrogenation, a conductor-insulator transition occurs when the electron Fermi energy reaches approximately 1. 1 electron volts, accompanied by a change in the Raman spectrum. This insulating state is fully reversible, with graphene regaining conductivity upon applying a voltage, and the Raman spectrum returning to its original state, enabling accurate estimation of the Fermi energy levels associated with both transitions.
Proton Transport Mechanisms in Graphene Materials
This research focuses on proton transport in two-dimensional materials, particularly graphene, and how this transport can be controlled and enhanced, crucial for applications in fuel cells, sensors, and energy storage technologies. Researchers employ electrochemical techniques, Raman spectroscopy, and other characterisation methods to understand the materials at a microscopic level. The research suggests that corrugations in graphene create pathways for faster proton transport, providing more surface area and potentially lowering energy barriers for proton movement. The type of electrolyte used significantly affects proton conductivity, with ionic liquids being investigated as promising candidates.
Computational modelling using Density Functional Theory provides valuable insights into the mechanisms of proton transport and helps to predict the properties of new materials. Graphene-based membranes are being explored for applications in separation and filtration, including the selective transport of protons. The research has significant implications for a wide range of applications, including more efficient fuel cells, highly sensitive sensors, and new types of energy storage devices. Graphene-based membranes could be used to purify water by selectively removing contaminants. The potential for using proton transport in graphene for creating memristors and neuromorphic computing devices is also being explored.
Electrochemical Hydrogenation, Graphene, and Isotope Effects
This research elucidates the mechanism behind the electrochemical hydrogenation of graphene, revealing a complex interplay of competing processes and the influence of material morphology. Scientists demonstrated that hydrogenation competes with the spontaneous formation of hydrogen molecules and proceeds significantly faster than hydrogenation using accelerated hydrogen atoms. Importantly, the reversal of this process, dehydrogenation, occurs through the oxidative desorption of protons. The team observed that substituting protons with deuterons lowers the potential required for hydrogenation and results in a more stable compound. The presence of concave regions within corrugated graphene, which resist full hydrogenation, governs the conductor-insulator transition of the material by maintaining localized electronic conductivity. The authors suggest future work could extend these principles to other ions, potentially enabling control of graphene’s electronic properties for novel chemistry-based computing devices.
👉 More information
🗞 Mechanism of the electrochemical hydrogenation of graphene
🧠 ArXiv: https://arxiv.org/abs/2510.19505