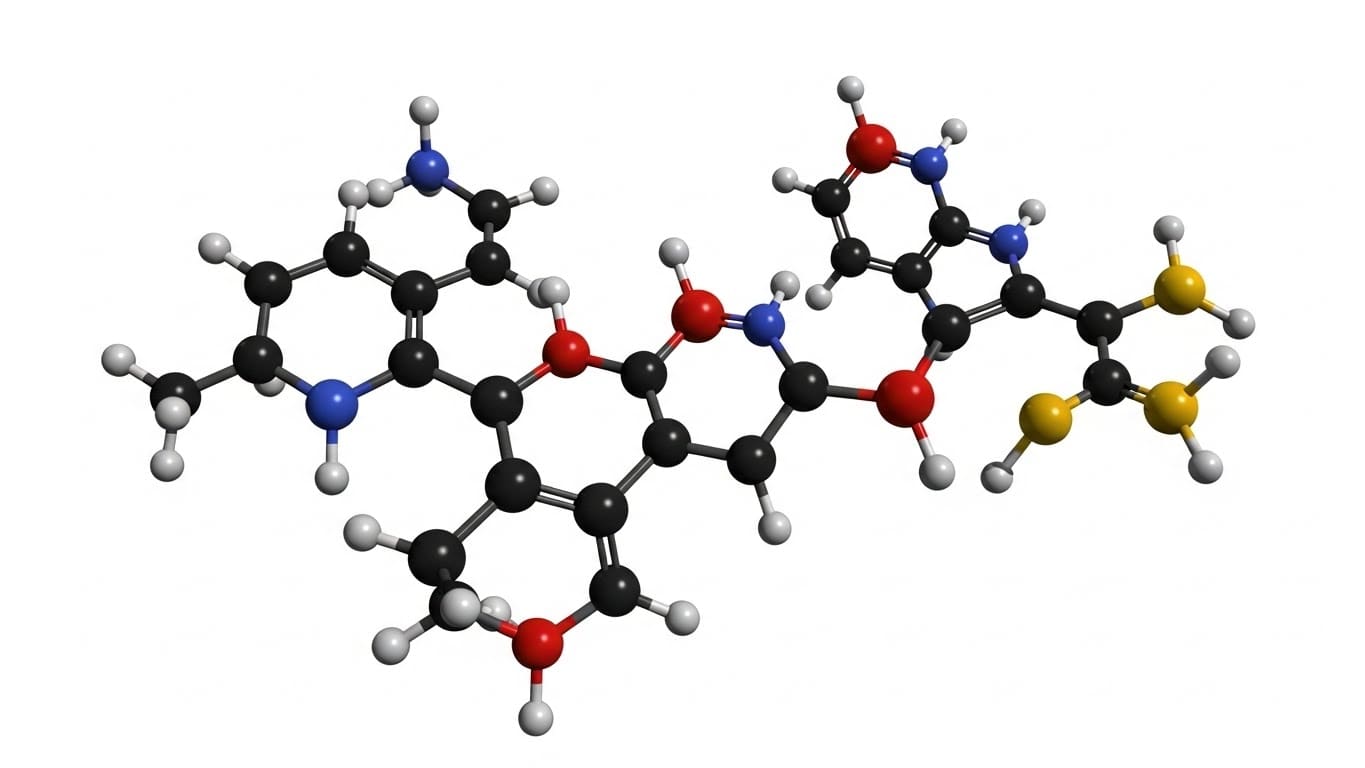
Polaris Quantum Biotech (PolarisQB) developed QuADD, the first drug discovery software platform built around quantum computing for lead identification. Utilizing a D-Wave Advantage system with over 5000 qubits, QuADD reduces drug design time from years to just hours. This platform can explore up to 10^30 theoretical molecules, optimizing for key properties and accelerating early-stage drug discovery.
Quantum Annealing Accelerates Early Drug Discovery
Quantum annealing offers a new approach to early drug discovery by rapidly exploring a vast chemical space—up to 10^30 theoretical molecules—and optimizing for critical properties like solubility and toxicity. The QuADD platform, leveraging a D-Wave system with over 5000 qubits, can achieve this in just hours, a significant acceleration compared to traditional methods and generative AI which may require weeks or months for similar results. This speed is achieved by solving complex optimization problems, adapting the Knapsack Problem to identify molecular fragments fitting specific binding sites while adhering to synthetic rules.
A recent comparison of QuADD with an AI-based system (BInD) demonstrated that, while both generated novel compounds, QuADD’s molecules exhibited superior binding affinities and more favorable drug-like characteristics. Specifically, QuADD-generated structures showed lower synthetic complexity, potentially reducing synthesis costs by a significant order of magnitude for a set of 100 compounds, and maintained strong interaction fidelity. This focus on actionable results—molecules likely to be synthesizable and effective—positions quantum annealing as a promising advancement in lead identification and optimization.
QuADD Platform Optimizes Molecules via QUBO Adaptation
This allows the system to simultaneously consider binding affinity, drug-like properties, and synthetic feasibility—all critical for viable drug candidates. By focusing on constraints during molecule generation, QuADD efficiently explores chemical space, reaching approximately 10^30 theoretical molecules to pinpoint promising leads. Compared to generative AI methods like BInD, QuADD prioritizes quality and “actionability” by generating molecules with superior binding affinities and lower synthetic complexity. A study showed QuADD generated 3,000 molecules in roughly 30 minutes, while BInD required approximately 40 hours using a standard GPU setup to achieve the same output. This focus on realistic, synthesizable compounds minimizes downstream filtering and validation, potentially accelerating the drug discovery process.
D-Wave Advantage System Enables 10^30 Molecule Exploration
The D-Wave Advantage system powers the QuADD platform, enabling the exploration of approximately 10^30 theoretical molecules for potential drug candidates. This vast chemical space is searched while simultaneously optimizing critical properties like permeability, solubility, and toxicity—all within a matter of hours. Compared to generative AI methods, QuADD focuses on generating a smaller number of high-quality, synthesizable molecules. The resulting compounds from QuADD demonstrate superior binding affinities and drug-like characteristics, reducing the risk of later-stage failures due to impracticality or poor efficacy.
QuADD Demonstrates Superior Binding Affinity to BInD
Though both systems created compounds that fit the binding site, QuADD’s molecules demonstrated superior binding affinity and stronger interaction fidelity—critical factors for effective drug action. This speed advantage allows for faster iteration in the early stages of drug discovery. Crucially, QuADD’s approach prioritizes “actionable” results, generating structures with lower synthetic complexity, better drug-like properties, and more realistic potential for synthesis. Compared to BInD’s focus on broad chemical diversity, QuADD confines its search to a chemically relevant space, leading to molecules likely to be both innovative and readily testable. The analysis showed substantially stronger predicted binding affinities for QuADD-generated compounds within the top 100 candidates.
While many metrics can be used to select a final set of candidates for synthesis in a drug discovery campaign, it is standard practice to use an estimate of binding affinity in the selection process (the higher the binding affinity, the more effective the drug).