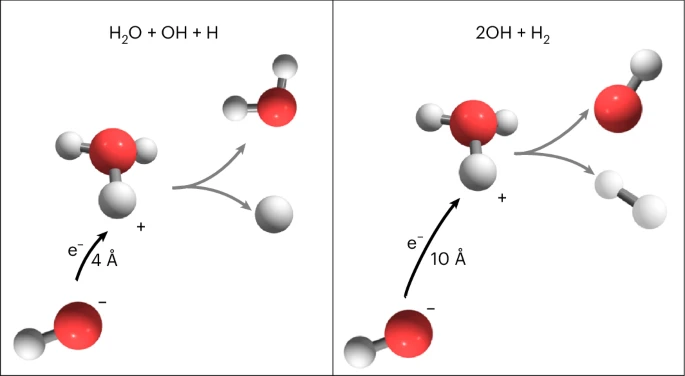
The provided references encompass a wide range of topics in ion chemistry and molecular dynamics, with a particular focus on isotope effects and dissociative recombination processes. These studies explore the behavior of ions such as H3O+ and D3O+, examining how isotopic substitution influences reaction pathways and rates through mechanisms like non-adiabatic transitions and vibrational mode changes. Experimental setups, including the DESIREE storage ring and SNICS source, enable precise observations of molecular dynamics under controlled conditions, shedding light on bond cleavage mechanisms in systems such as H3+ and D3+.
The research also delves into sequential versus concerted dissociation pathways, contributing to understanding fundamental chemical processes in diverse environments, from interstellar clouds to biological systems. These investigations collectively enhance our knowledge of reaction mechanisms and molecular behavior across various contexts.
Ion Dynamics
The dynamics of H3O+ ions are significantly influenced by isotope effects, particularly in their vibrational modes and reaction pathways. Isotopic substitution alters the mass distribution within the molecule, which directly impacts its vibrational frequencies and bond-breaking mechanisms. Experimental studies have shown that these changes can lead to variations in dissociation rates and product distributions during ion-molecule reactions.
Quantum mechanical effects, such as non-adiabatic transitions, play a critical role in determining the reaction dynamics of H3O+ ions. These effects are particularly pronounced when isotopic substitution occurs, as it modifies the potential energy surfaces and coupling between vibrational modes. Theoretical models have been developed to describe these phenomena, providing insights into the interplay between isotope effects and reaction mechanisms.
Advanced experimental techniques, including dissociative recombination experiments and ion storage ring studies, have facilitated the study of H3O+ isotope effects. These methods allow for precise measurements of reaction rates and product distributions under controlled conditions, enabling researchers to disentangle the contributions of isotopic substitution from other factors. Such studies are particularly relevant for understanding molecular chemistry in interstellar environments, where H3O+ ions play a significant role.
Isotope Effects
Isotopic substitution significantly impacts the vibrational frequencies and coupling between modes in H3O+ ions. This effect is critical for understanding reaction dynamics, as it influences bond-breaking processes and overall reactivity. Advanced detection systems, such as imaging detectors and time-resolved spectroscopy, are employed to capture these effects with high precision.
Theoretical models complement experimental findings by simulating potential energy surfaces and non-adiabatic transitions. These simulations provide a deeper understanding of how isotopic substitution influences reaction mechanisms. By combining experimental data with theoretical insights, researchers can better interpret observations in interstellar environments and other settings where H3O+ ions are prevalent.
Molecular Dissociation Mechanisms
The dissociation of H3O+ ions is heavily influenced by isotope effects, which alter vibrational modes and reaction pathways. Isotopic substitution modifies the coupling between vibrational modes, impacting bond-breaking processes and product distributions. Experimental studies using ion storage rings and dissociative recombination techniques have provided valuable insights into these mechanisms.
Quantum mechanical effects, such as non-adiabatic transitions, further complicate the dynamics of H3O+ ions. These effects are particularly significant in interstellar environments, where H3O+ plays a key role in molecular chemistry. By leveraging advanced imaging systems and time-resolved measurements, researchers can capture the outcomes of these processes with high precision.
Imaging Systems and Time-Resolved Measurements
Imaging detectors and time-resolved spectroscopy are essential tools for studying the dynamics of H3O+ ions. These systems enable precise measurements of reaction rates and product distributions under controlled conditions, providing critical data for understanding isotope effects and quantum mechanical influences.
Theoretical models, which simulate potential energy surfaces and non-adiabatic transitions, complement experimental findings. By combining these approaches, researchers can gain a comprehensive understanding of how isotopic substitution impacts reaction mechanisms in H3O+ ions. Such insights are particularly valuable for interpreting observations in interstellar environments and other astrophysical settings.
Interstellar Chemistry
H3O+ ions play a significant role in molecular chemistry within interstellar environments. Their dynamics, influenced by isotope effects and quantum mechanical processes, contribute to the formation of complex molecules. Advanced experimental techniques, such as ion storage ring studies and dissociative recombination experiments, are crucial for understanding these mechanisms.
Theoretical models, which simulate potential energy surfaces and non-adiabatic transitions, provide additional insights into the role of H3O+ ions in interstellar chemistry. By combining experimental data with theoretical simulations, researchers can better interpret observations and predict the behavior of H3O+ ions under various conditions. These studies are essential for advancing our understanding of molecular processes in space.
More information
External Link: Click Here For More