Artificial intelligence agents represent a potentially revolutionary approach to drug discovery, offering the capacity to automate and accelerate complex research processes. Srijit Seal, Dinh Long Huynh, and Moudather Chelbi, along with colleagues including Sara Khosravi and Ankur Kumar at Mattson Thieme’s institution, present a comprehensive overview of these agentic AI systems, which combine large language models with tools for perception, computation, and action. This work details how these agents can integrate diverse biomedical data, execute experiments, and refine hypotheses autonomously, demonstrably compressing research timelines from months to hours while maintaining scientific rigour. Importantly, this represents the first detailed examination of real-world implementations and quantifiable benefits of agentic AI actively deployed in operational drug discovery settings, paving the way for faster, more efficient development of new medicines.
AI Accelerates Drug Discovery and Toxicology
This document details the application of artificial intelligence (AI), specifically large language models and agentic AI, to accelerate drug discovery and investigative toxicology. It explores both the potential benefits and challenges of integrating AI into these complex scientific fields, going beyond theoretical possibilities to examine practical design considerations, data characteristics, and successful implementations. At the core of this advancement is agentic AI, which moves beyond simple predictive models to create systems capable of autonomously planning, executing, and learning. These agents actively perform tasks such as searching databases, designing experiments, analysing data, and generating reports.
Large language models serve as central orchestrators, providing natural language understanding, reasoning, and planning capabilities. A crucial technique employed is Retrieval-Augmented Generation (RAG), which allows agents to access and incorporate up-to-date information from external databases, improving accuracy and relevance. The research emphasizes the need for AI systems to handle diverse data types, including chemical structures, genomic data, images, text, and quantitative measurements. Knowledge graphs are used to represent complex relationships between biological entities, facilitating reasoning and discovery.
The concept of creating digital twins of biological systems and laboratory processes is explored, enabling AI agents to simulate experiments and optimize workflows. Detailed analysis reveals the challenges posed by data used in drug discovery and toxicology, including incompleteness, bias, variability, and the need for quantitative context. Combining computational and experimental approaches is highlighted, with AI agents used to prioritize experiments and optimize workflows, reducing time and cost. The document begins with an introduction and overview, then delves into design considerations for agentic AI, data characteristics, and challenges.
It presents case studies demonstrating how AI agents are being used to address specific challenges in drug discovery and toxicology, providing insights into the design and implementation of successful AI systems. The case studies demonstrate significant achievements, including rapid literature review for molecular prioritization, accurate toxicity predictions, a 400-fold reduction in qPCR workflow cycle time, and a compression of preclinical workflows from weeks to hours. Other successes include identifying potential drug candidates for rare diseases, automating small molecule synthesis, generating novel targets in the Wnt signaling pathway, and identifying promising biopharmaceutical assets.
Agentic AI for Biomedical Data Analysis
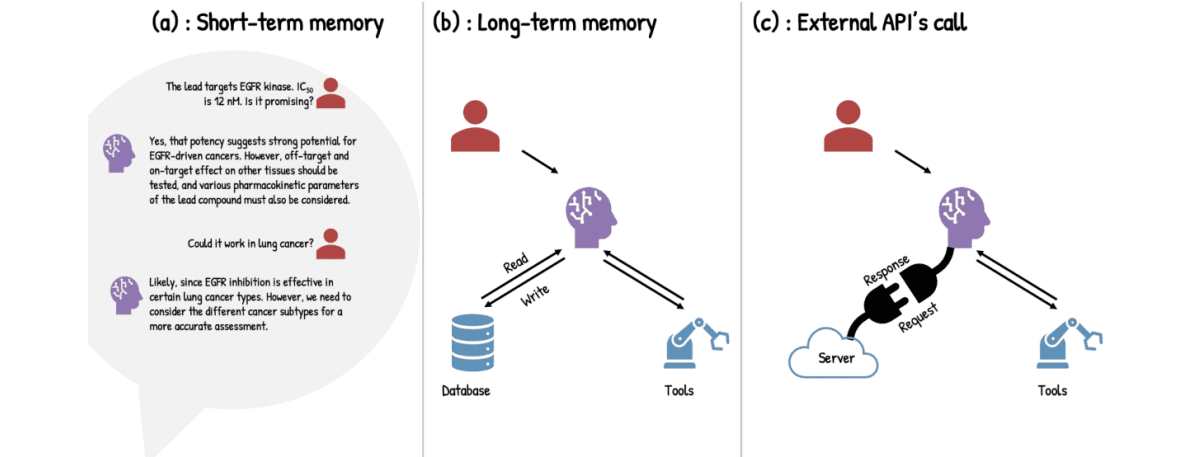
Researchers have pioneered the application of agentic artificial intelligence to drug discovery, building upon recent advances in large language models and coupling them with external tools, data sources, and memory. These engineered systems demonstrate autonomous reasoning, action, and learning throughout complex research workflows, moving beyond passive predictive or generative models. At the core of these agentic systems are large language models, augmented with four key tool types designed to overcome limitations of standalone models. Perception tools function as an augmentation layer, enabling the system to gather information from diverse biomedical databases including ChEMBL, PubChem, STRING, and Reactome.
Computation tools facilitate predictions, simulations, and data analysis, often serving as wrappers for pre-trained models like AlphaFold or data processing pipelines such as NextFlow, and typically execute on local computers or cloud-based high-performance computing platforms to handle large-scale data. Action tools provide the agentic system with the ability to interact with the physical world, connecting to robotic pipetting systems, automated cell-based assays, and next-generation sequencing library preparation to close the loop between in silico design and empirical validation. Finally, memory tools maintain persistence across interactions, allowing the agentic system to learn and refine its approach over time. The study demonstrates how these integrated tools enable the agentic AI to perform tasks such as literature review, toxicity prediction, specifically liver and cardiotoxicity, experimental planning, and interfacing with lab automation. This approach compresses workflows that previously took months into hours while maintaining scientific traceability, representing a substantial gain in speed, reproducibility, and scalability. Researchers successfully implemented these systems to address key stages of drug research, from target discovery and iterative Design-Make-Test-Analyze cycles to preclinical safety assessment and drug repurposing.
Agentic AI Accelerates Drug Discovery Pipelines
Researchers have achieved a significant breakthrough in drug discovery by developing agentic artificial intelligence (AI) systems capable of autonomously performing complex research workflows. This work represents the first comprehensive demonstration of agentic AI deployed in operational drug discovery settings, demonstrating substantial gains in speed, reproducibility, and scalability, compressing workflows that once took months into hours while maintaining scientific traceability. The core of this advancement lies in coupling large language models with external tools, data sources, and memory, creating systems that can ‘think’, ‘act’, ‘observe’, and ‘reflect’ in iterative loops. These agentic systems utilize four key tool types to implement end-to-end pipelines.
Perception tools gather information from biomedical databases such as ChEMBL, PubChem, and Reactome, integrating both structured and unstructured data. Computation tools enable predictions, simulations, and data analysis, often utilizing pre-trained models like AlphaFold or data processing pipelines like NextFlow, and frequently operate on cloud high-performance computing platforms to handle large-scale data. Action tools connect to robotic pipetting, automated cell-based assays, and next-generation sequencing, closing the loop between in silico design and empirical validation. Finally, memory tools maintain persistence across tasks by storing, retrieving, compressing, and updating the agent’s working knowledge, capturing valuable patterns and toxicity findings for repeated use.
The most basic architecture, the ReAct agent, enables the large language model to dynamically select and execute the necessary tools when receiving tasks. This allows the system to take evidence, form a hypothesis, transform that hypothesis into predictions, test those predictions experimentally, and retain what is learned from previous cycles. Researchers demonstrate how these systems can autonomously perform tasks across the entire drug discovery pipeline, from target discovery and DMTA cycles to preclinical safety and drug repurposing, reshaping current practice. This innovative approach promises to accelerate the pace of drug development and reduce associated costs.
AI Accelerates Drug Discovery and Business Development
This work demonstrates a multi-agentic artificial intelligence system capable of accelerating drug discovery and development, from initial candidate selection through to business development and potential partnerships. By integrating diverse data sources, including structured databases and unstructured text from scientific literature and market reports, the system constructs a comprehensive knowledge graph that underpins scientific, clinical, and strategic analyses. This allows for rapid assessment of drug candidates, prediction of clinical trial risks, and formulation of effective business strategies, ultimately delivering data-driven recommendations for advancing promising compounds. The team successfully deployed this system within a pharmaceutical company to triage its portfolio, achieving significant gains.
👉 More information
🗞 AI Agents in Drug Discovery
🧠 ArXiv: https://arxiv.org/abs/2510.27130