Understanding how immune cells move is crucial for investigating a range of diseases, and researchers are increasingly using advanced microscopy to track these movements, generating vast amounts of image data. To tackle the challenges of storing and transmitting these large datasets, Xiaowei Xu, Justin Sonneck from Leibniz-Institut für Analytische Wissenschaften, Hongxiao Wang from Capital Normal University, and colleagues developed FlowRoI, a new framework for efficiently compressing images of migrating cells. FlowRoI identifies areas of movement within each frame and focuses compression on these regions of interest, significantly reducing file sizes without sacrificing image quality. The team demonstrates that FlowRoI achieves compression rates 2. 0 to 2. 2times higher than standard methods while maintaining detailed cellular images, offering a powerful tool for high-throughput analysis of immune cell migration and accelerating potential clinical applications.
Optical Flow Guides Image Compression of Cell Motion
This research introduces FlowROI, a new method for compressing images generated by microscopy, specifically designed for analysing the movement of immune cells. Large-scale microscopy experiments produce massive amounts of image data, and compressing this data efficiently without losing crucial information about cell movement presents a significant challenge. FlowROI addresses this by leveraging optical flow, a technique that detects motion between consecutive frames, to identify regions of interest (ROIs) covering moving cells. This focuses compression on the important parts of the image, achieving 2.
0 to 2. 2times higher compression compared to standard JPEG2000 at the same image quality. The method preserves segmentation performance, achieving comparable accuracy in identifying and segmenting cells while significantly reducing storage space required for large-scale microscopy datasets. FlowROI proves practical for real-world applications due to its speed, simplicity, and lack of training requirements.
FlowRoI For Fast Immune Cell Tracking
The study pioneers FlowRoI, a fast region of interest (ROI) extraction framework designed to compress images generated by high-throughput microscopy of immune cell migration. Researchers addressed the challenge of exponentially increasing data volumes by focusing compression efforts on biologically relevant areas within images, significantly reducing storage and transmission burdens. The method operates by computing optical flow from pairs of consecutive frames, revealing local motion patterns of migrating cells. This optical flow data drives the derivation of ROI masks, effectively highlighting cell-containing regions critical for downstream analysis such as segmentation and tracking.
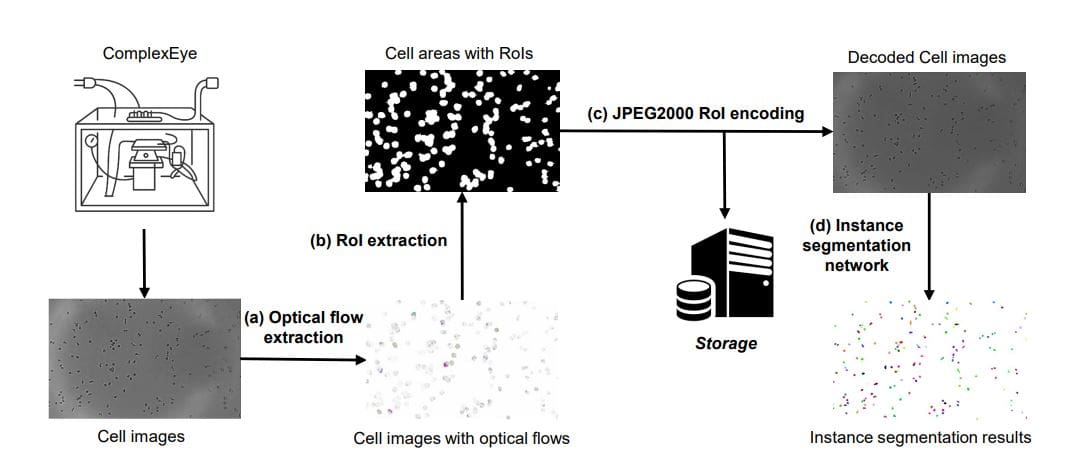
The framework consists of four stages: optical flow computation, ROI extraction, JPEG2000 ROI encoding, and final downstream analysis. Optical flow extraction employs a three-step refinement process involving denoising, stabilization, and flow estimation, ensuring robust and accurate flow fields. ROI extraction utilizes a saliency-based segmentation procedure, constructing a motion-saliency map and applying a threshold to identify cell-containing regions. Finally, JPEG2000 ROI encoding prioritizes ROI pixels with lower compression, while background pixels receive higher compression, optimizing data size without sacrificing fidelity in biologically meaningful areas.
FlowRoI Compresses Cell Migration Microscopy Data
Scientists have developed FlowRoI, a new framework for compressing images generated by high-throughput microscopy, specifically designed for studying the movement of immune cells. This work addresses the challenge of managing the vast amounts of data produced by advanced microscopes capable of capturing high-resolution videos of cell migration. The team achieved significant improvements in both compression efficiency and image quality using this innovative approach. FlowRoI operates by first estimating the motion of cells between consecutive images, creating a detailed map of cellular activity. This motion data is then used to identify regions of interest, or ROIs, which reliably cover nearly all migrating cells within the image.
The system then encodes both the original image and the ROI mask using JPEG2000, prioritizing the ROI pixels for higher fidelity while compressing the background more aggressively. Experiments demonstrate that FlowRoI achieves 2. 0 to 2. 2times higher compression rates at matched peak signal-to-noise ratio (PSNR) compared to standard JPEG2000 compression. The framework operates with high efficiency, reaching an average throughput of approximately 30 frames per second on a laptop equipped with an Intel i7-1255U CPU. Researchers validated the effectiveness of FlowRoI by assessing its impact on downstream analysis, specifically cell instance segmentation, demonstrating that FlowRoI preserves image details critical for accurate segmentation.
FlowRoI Compresses Microscopy Data Efficiently
Researchers have developed FlowRoI, a new method for compressing images generated by high-throughput microscopy, specifically designed for studying the movement of immune cells. This technique addresses the challenge of managing the large volumes of data produced when tracking cells over time, offering a practical solution for large-scale experiments. FlowRoI identifies regions of interest within each image frame by analysing motion, effectively focusing compression on the areas where cells are actively migrating. The method achieves compression rates 2. 0 to 2. 2times higher than standard techniques while maintaining equivalent image quality, or conversely, delivers superior image quality at the same compression level. Importantly, FlowRoI operates efficiently on standard computer hardware, processing approximately 30 frames per second on a typical laptop, and requires minimal parameter adjustment for robust performance.
👉 More information
🗞 FlowRoI A Fast Optical Flow Driven Region of Interest Extraction Framework for High-Throughput Image Compression in Immune Cell Migration Analysis
🧠 ArXiv: https://arxiv.org/abs/2511.14419