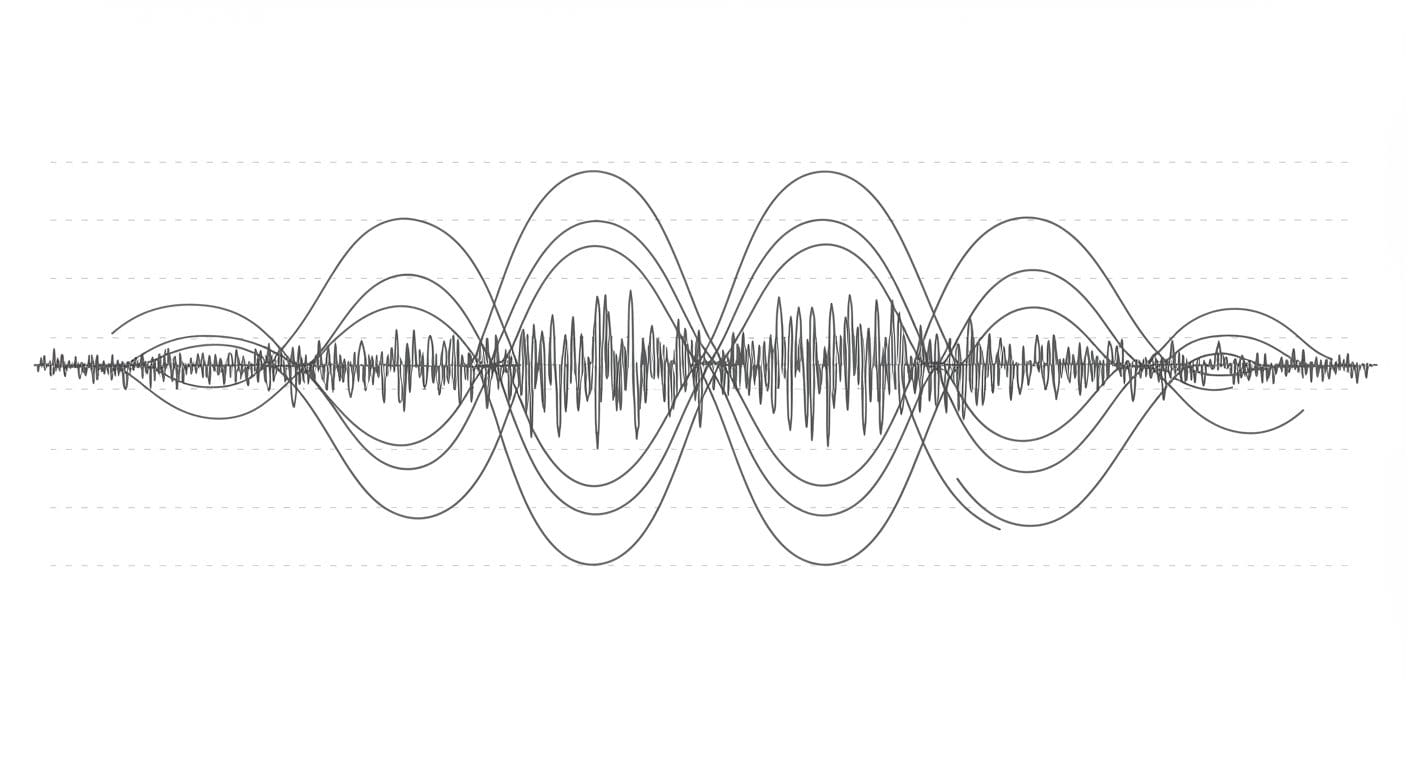
Electromagnetic biomaterial imaging stands to gain a significant boost from a new technique that maps tissue properties with unprecedented sensitivity, as demonstrated by Alessandro Settimi from MIM, USR-Lazio, IIS “Sandro Pertini” and colleagues. Their research introduces Quantum Phase Space Tomography (QPST), a method that probes the full phase space of electromagnetic fields interacting with tissue, revealing subwavelength features often lost in conventional imaging. The team couples analytical modelling of tissue with advanced measurement techniques and Bayesian inference to recover key physiological parameters, such as layer thickness and dispersion, and even to quantify microstructural heterogeneity indicative of conditions like malignancy. By leveraging state-of-the-art technologies and innovative algorithms, QPST promises a new paradigm for non-invasive medical imaging, bridging fundamental electromagnetic theory with emerging technologies to deliver detailed insights into tissue composition and structure.
Tissue Impedance Analysis for Body Composition
This document details a comprehensive analysis of bioelectrical impedance, focusing on how electrical current travels through different tissues like fat and muscle. The research aims to accurately estimate body composition, including fat mass, muscle mass, and water content, and to characterize the unique electrical properties of these tissues. The analysis considers how impedance changes with varying electrical frequencies, crucial for precise measurements. The document meticulously examines tissue-specific parameters and tolerances for both fat and muscle, detailing how impedance values and current penetration depths vary.
It also explores the design of electrodes, considering both spherical and cylindrical shapes, and how their size and spacing impact measurement accuracy. Different electrode configurations, such as Wenner’s and square arrangements, are also investigated to optimize signal quality. A key finding is the importance of frequency selection, as different frequencies are better suited for targeting specific tissues and revealing meaningful information. Understanding how deeply electrical current penetrates into tissues is also critical for ensuring measurements accurately reflect the volume being assessed.
This detailed modeling and analysis highlights the significant impact of electrode design and configuration on measurement precision. This research has broad applications, including accurate body composition analysis, potential medical diagnostics for detecting tissue changes, and physiological monitoring of hydration status. It also offers valuable insights for fitness and wellness assessments, and provides a foundation for advanced bioimpedance spectroscopy, a technique used to characterize tissue properties in detail. This innovative approach moves beyond traditional methods that rely on mapping scalar permittivity, offering a more detailed and sensitive analysis. The technique begins with a precise analytical model representing tissue as layered, skin, fat, muscle, and bone, and uses the quasi-static Helmholtz equation to describe electrical potential within each layer. To probe tissue characteristics, scientists engineered a structured electromagnetic probe, such as a squeezed microwave pulse, and developed a measurement apparatus to perform complete quantum tomography of the field after it interacts with the tissue.
This process meticulously characterizes the outgoing field’s Wigner function, a quantum mechanical representation of the field’s phase-space distribution, revealing sub-wavelength features and subtle correlations often lost in conventional imaging. The team then implemented a Bayesian inferential engine, projecting the measured data onto the analytically derived tissue response manifold to accurately recover key physiological parameters, including layer thickness and dispersion characteristics. A crucial innovation is the development of the Dielectric Anaplasia Metric (DAM), a quantitative biomarker for tissue irregularity that quantifies microstructural heterogeneity, such as malignancy, by analyzing deviations in key parameters. Scientists harnessed state-of-the-art quantum sensors, specifically nitrogen-vacancy (NV) diamond magnetometers, offering exceptional sensitivity and subcellular resolution. Furthermore, the team integrated physics-informed neural networks and diffusion priors to address the challenges of reconstructing the tissue’s quantum state, ensuring robust and accurate results. This innovative approach leverages a rigorous forward model based on Maxwell’s equations and the Cole-Cole model to describe layered tissue, enabling precise characterization of dielectric properties. The team prepares a specially designed electromagnetic probe, such as a squeezed microwave pulse, and then performs complete quantum tomography of the outgoing field after it interacts with tissue. Experiments reveal that QPST recovers the Wigner quasi-probability distribution, capturing subwavelength features and non-classical correlations lost in conventional imaging methods.
By projecting the measurement data onto the analytically derived tissue response manifold, scientists can accurately recover key physiological parameters, including layer thickness and dispersion characteristics. The method further defines a Dielectric Anaplasia Metric (DAM) that quantifies tissue microstructural heterogeneity, offering a potential new biomarker for malignancy via deviations in Cole-Cole parameters. The breakthrough delivers a powerful analytical framework that constrains the inversion process, ensuring solutions remain physically plausible and avoiding unphysical results. By embedding the forward equations directly into physics-informed neural networks or utilizing Bayesian optimization, the team accelerates inference and enhances robustness. Data confirms that QPST can accurately link the dominant Cole-Cole relaxation times to low and high-frequency impedance ratios, providing a quantitative measure of tissue characteristics. By combining a rigorous forward model based on Maxwell’s equations and the Cole-Cole model with advanced quantum sensing and Bayesian inference, the method reconstructs the full Wigner quasi-probability distribution of the electromagnetic field interacting with tissue. This allows for the recovery of key physiological parameters, such as layer thickness and dispersion, and the quantification of tissue microstructural heterogeneity through a newly defined Dielectric Anaplasia Metric. The results demonstrate the potential to non-invasively map tissue permittivity with unprecedented sensitivity, potentially enabling the detection of anomalies at the cellular level.
The authors acknowledge limitations inherent in the complexity of biological tissues and the challenges of real-time data inversion. Future research will focus on validating the method using realistic phantoms and extending its capabilities to three-dimensional imaging and incorporating plasmonic enhancements. Combining the analytic model with machine learning algorithms, such as physics-informed neural networks, is also crucial for achieving real-time image reconstruction. Ultimately, QPST represents a significant step towards a new paradigm for medical imaging, bridging fundamental electromagnetic theory with emerging technologies to offer a powerful tool for non-invasive diagnostics.
👉 More information
🗞 Quantum Phase Space Tomography for Electromagnetic Biomaterial Imaging
🧠 ArXiv: https://arxiv.org/abs/2509.00534