Proton-coupled electron transfer rates, crucial in electrocatalysis, are accurately calculated using a new electrochemistry-driven dynamics approach. This method realistically models reaction pathways under constant potential, revealing nuclear quantum effects significantly impact reaction rates, exceeding one order of magnitude for the hydrogen evolution reaction’s Volmer step.
The efficiency of electrocatalysis, central to technologies ranging from hydrogen production to carbon dioxide reduction, hinges on the precise choreography of proton-coupled electron transfers (PCET), where the movement of protons and electrons are intimately linked. Accurately modelling the rates of these PCET reactions proves difficult, particularly when accounting for nuclear quantum effects (NQEs), which arise from the wave-like behaviour of atomic nuclei. These effects can significantly alter reaction dynamics, yet are computationally demanding to incorporate. Researchers led by Li Fu, Yifan Li, Menglin Sun, Xiaolong Yang, Bin Jin, and Shenzhen Xu, from Peking University and Princeton University, present a novel computational approach to address this challenge. Their work, detailed in ‘Electrochemistry-Enhanced Dynamic Paths Sampling Unveiling Nuclear Quantum Effects in Electrocatalysis’, introduces a method for realistically sampling reaction pathways under constant potential conditions, revealing that NQEs can exert an impact exceeding one order of magnitude on the rate constants governing the Volmer step of the hydrogen evolution reaction. This demonstrates the essential role of NQEs in electrochemical processes.
Researchers present a computational methodology for accurately modelling proton-coupled electron transfer (PCET) reactions, crucial steps in electrocatalysis, which addresses the longstanding challenge of calculating reaction rates while accounting for nuclear quantum effects (NQEs) under realistic, constant potential conditions. PCET involves the simultaneous transfer of both protons and electrons, and NQEs arise from the wave-like behaviour of atomic nuclei, becoming significant at low temperatures or for light atoms like protons. The new approach accurately models the Volmer step, the initial hydrogen adsorption stage, of the hydrogen evolution reaction, demonstrating that NQEs can influence reaction rates by more than one order of magnitude, underlining the necessity of their inclusion in reliable electrochemical modelling.
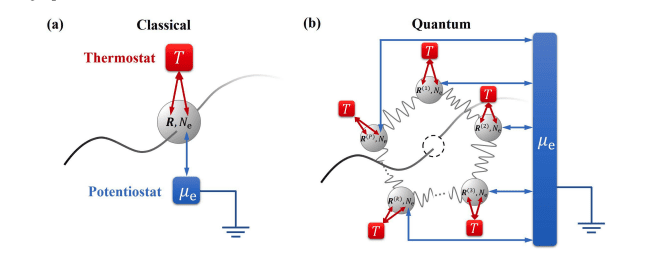
The developed electrochemistry-driven dynamics approach overcomes limitations inherent in traditional rate constant calculations, which often struggle with rare-event sampling, where the probability of observing a reaction is very low. By avoiding the need to pre-define reaction coordinates – specific pathways for the reaction to occur – the method facilitates realistic enhanced path sampling under constant potential conditions, offering a more accurate representation of electrochemical dynamics. This is achieved through an integrated workflow combining density functional theory (DFT), a quantum mechanical method used to calculate the electronic structure of materials, with deep potential molecular dynamics (DeePMD). DeePMD employs machine learning to create accurate and efficient interatomic potentials, enabling long-timescale simulations.
Researchers utilise software packages such as VASP, a widely used DFT code, DeePMD-Kit, and DP-Compress to implement the methodology. Initial electronic structures are established using DFT calculations, and molecular dynamics simulations are accelerated using the machine-learned DeePMD potentials. The iterative refinement of the deep potential through active learning, where the model is continuously improved based on simulation results, enhances the accuracy of the molecular dynamics simulations, providing a robust and reliable model for studying electrocatalytic reactions.
The research leverages the Perdew-Burke-Ernzerhof (PBE) functional, a common approximation used within DFT to calculate the exchange-correlation energy, alongside DFT-D3 dispersion corrections to accurately model material interactions, including van der Waals forces, which are crucial for describing surface adsorption and interactions. Dipole corrections applied to surface calculations mitigate spurious electric fields that can arise from the finite size of the simulation cell, further refining the accuracy of the model and ensuring the reliability of the simulations.
Simulations utilise carefully constructed supercells, large repeating units of the material, and rigorous convergence criteria, ensuring that the results are statistically significant and reliable. Researchers employ the grand canonical constant potential approach to accurately simulate the electrochemical interface, maintaining a constant electrochemical potential during the simulations, which reflects realistic conditions found in electrochemical devices. This provides a more realistic and comprehensive understanding of PCET mechanisms in electrocatalysis, offering insights essential for the design of improved electrocatalytic materials and processes.
👉 More information
🗞 Electrochemistry-Enhanced Dynamic Paths Sampling Unveiling Nuclear Quantum Effects in Electrocatalysis
🧠 DOI: https://doi.org/10.48550/arXiv.2506.16807