Electrocorticographic brain-computer interfaces hold immense promise for both fundamental neuroscience and advanced clinical treatments, but current devices present significant limitations in balancing coverage area, electrode density, and surgical risk. Tao Zou, Na Xiao, and Ruihong Weng, alongside colleagues from The University of Hong Kong and The Chinese University of Hong Kong, now demonstrate a novel solution to these challenges. The team developed a flexible, high-density electrocorticogram (ECoG) array that utilises a guidewire-driven deployment method, allowing for minimally invasive implantation through small skull openings. This innovative device, packing 256 electrodes into a compact area, successfully captures detailed auditory neural signals in canine models and achieves over 80% accuracy in sound classification using standard machine learning techniques, representing a substantial step towards resolving the key trade-offs that currently limit the potential of brain-computer interfaces.
Electrocorticography for High Precision Brain Interfaces
Electrocorticography (ECoG) involves placing electrodes directly on the brain’s surface to record electrical activity, offering greater precision than electroencephalography (EEG). Researchers are developing ECoG-based brain-computer interfaces (BCIs) to restore function and improve the lives of individuals with neurological disorders, aiming to decode brain signals to control prosthetic limbs, reconstruct speech, and identify emotional states. Current research focuses on improving ECoG technology through minimally invasive implantation techniques and enhanced signal quality. Scientists are exploring soft, flexible electrodes that conform to the brain’s surface, robotic systems for precise electrode placement, and biodegradable electrodes.
Advanced signal processing algorithms, spatial filtering, and frequency analysis are employed to extract meaningful information from brain activity and ensure long-term stability and biocompatibility. Materials science plays a significant role, with investigations into polymers like polyimide and Parylene C, as well as two-dimensional materials such as graphene, for creating flexible and biocompatible electrodes. Manufacturing techniques like 3D printing and microfabrication are used to create customized implants and surgical guides. Researchers are refining implantation procedures to minimize surgical risks and brain tissue damage, utilizing minimally invasive approaches and advanced imaging.
Studies utilize animal models, including non-human primates and minipigs, to test and refine these technologies. Data analysis involves sophisticated signal processing, spatial analysis, time-frequency analysis, and machine learning algorithms to decode brain signals and control external devices. Key concerns include biocompatibility, minimizing surgical risks, improving signal quality, and addressing the ethical implications of BCI technology.
Minimally Invasive High-Density Neural Interface Deployment
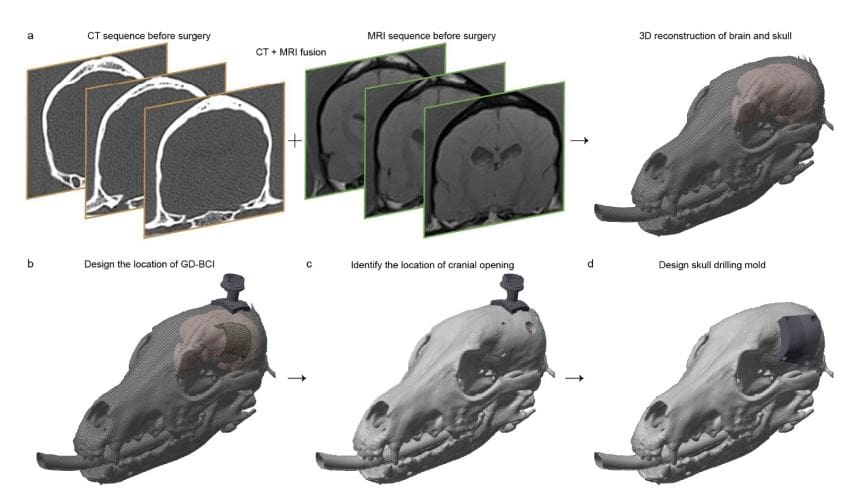
Scientists have engineered a new electrocorticographic brain-computer interface (BCI) designed for minimally invasive implantation and high-density neural recording. The device utilizes a guidewire-driven deployment technique, allowing insertion through small openings in the skull and seamless unfolding onto the brain’s dura mater, significantly reducing risks associated with traditional BCI implantation. The resulting device packs 256 electrodes into a 4 cm² area, achieving an electrode density of 64 electrodes per cm². Fabrication of the ultra-flexible electrode array involved photolithography to create a thin-film structure consisting of polyimide insulators, gold and chromium electrodes, and a nickel marker for MRI compatibility.
The array exhibited reliable signal quality, with 97. 7% of electrodes displaying impedance values below 100 kΩ at 1kHz. Perforations were introduced into the polyimide substrate to enhance conformability and promote stable contact with the brain surface. The final array measures just 21μm thick and possesses sufficient tensile strength for implantation. To connect the high-channel-count array to signal acquisition hardware, scientists utilized a commercially available land grid array (LGA) and a circular printed circuit board.
An interconnect extension was added for manoeuvrability during surgery. Recognizing the limitations of standard skull-mounted pedestals, the team designed and 3D-printed a lightweight, medical-grade resin pedestal tailored to each patient’s anatomy using computerized tomography (CT) scans. Rigorous testing confirmed the device’s structural resilience, demonstrating it could withstand forces between 1. 70 and 1. 90 N.
Mechanical simulations and experiments on artificial brain models revealed that any potential failure would occur at the junction between the guidewires and the array’s edge, preserving the integrity of the electrode pads. Deployment on the artificial brain model resulted in minimal disruption to signal quality, with impedance increases exceeding 1 MΩ for only 1. 95% of the channels.
High-Resolution Neural Signals From Minimally Invasive Implants
This research presents a groundbreaking electrocorticographic brain-computer interface (BCI) designed for minimally invasive implantation and high-resolution neural signal capture. Scientists developed a guidewire-driven device featuring an ultra-flexible electrode array containing 256 electrodes packed into an area of 4 cm². This array can be deployed epidurally through millimetre-sized skull openings and seamlessly unfolded onto the brain’s dura mater, addressing key limitations of current BCI technology. Experiments conducted on canines demonstrate the device’s ability to capture abundant, high-quality auditory neural signals.
Researchers recorded distinct auditory evoked potentials (AEPs) with amplitudes reaching up to 100 μV at approximately 50 milliseconds after stimulus onset, indicating rapid and sensitive detection of early-stage auditory processing. Mapping these signals revealed a clear spatial pattern of AEP response, with larger amplitudes localized to the auditory cortex. Detailed analysis of the neural signals revealed a wide bandwidth of activity, from slow waves to fast oscillations, with the γ2 frequency band (80-150Hz) exhibiting the most localized response, spanning approximately 4mm. Mapping brain responses to pure tones at 100Hz, 1000Hz, and 10000Hz revealed a clear frequency-dependent pattern of neural activation, with distinct features in spike duration, peak-to-peak amplitude, and activation location.
To demonstrate the potential for decoding auditory information, scientists developed a machine learning pipeline. Using signals from individual trials, they achieved 80. 3% accuracy in classifying sound frequencies using a Support Vector Machine classifier. This breakthrough demonstrates the potential to improve prostheses for patients unable to use cochlear implants and to decode neural activity for applications such as restoring speech. The extracted features proved useful across several standard machine learning models, validating the robustness of the approach.
High-Density Brain Signals via Minimally Invasive Device
This research demonstrates a new approach to electrocorticography, achieving high-density and large-area brain signal recording through a minimally invasive procedure. Scientists developed a guidewire-driven device capable of being inserted through small openings in the skull and unfolded onto the brain’s surface. The resulting electrode array, containing 256 electrodes within an area of 4 cm², successfully captured high-quality auditory neural signals in canine subjects. These signals exhibited distinct features related to hearing and enabled accurate classification of sound types with over 80% accuracy using established machine learning techniques.
👉 More information
🗞 Guidewire-driven deployment of high density ECoG arrays for large area brain-computer interface
🧠 ArXiv: https://arxiv.org/abs/2511.12907